 |
 |
- Search
| Asian Spine J > Volume 12(4); 2018 > Article |
|
Abstract
Purpose
To examine the relationship between postoperative bowel bladder disorder (BBD) and the efficacy of needle electrodes for the external anal sphincter (EAS) in intraoperative spinal cord monitoring with transcranial muscle action potentials (Tc-MsEP).
Overview of Literature
Spinal surgery for spina bifida, spinal cord tumor, and spinal tethered cord syndrome has a high rate of postoperative BBD. Monitoring of the EAS with Tc-MsEP is frequently performed during spinal surgery. We initially used plug-surface electrodes for this purpose, but have more recently switched to needle electrodes for the monitoring of the EAS. To date, there has been no comparison between the utility of these electrodes.
Methods
Waveform derivation, exacerbation of postoperative BBD, and sensitivity and specificity for prediction of BBD by 70% amplitude reduction of EAS activity using needle and plug-surface electrodes were investigated in 239 spine surgeries. The cut-off for the % drop in amplitude for BBD prediction was determined for EAS monitoring using a needle electrode.
Results
The overall rate of postoperative BBD aggravation was 7.1% (17/239 cases), with the individual rates using needle and plug-surface electrodes being 6.9% (8/116) and 7.3% (9/123), respectively. The waveform derivation rate was significantly higher using needle electrodes (91.3% [106/116] vs. 76.4% [94/123], p <0.01). In patients with baseline waveform detection, the sensitivity and specificity for postoperative BBD were similar in the two groups. With needle electrodes, a cutoff amplitude of Tc-MsEP for the EAS at the end of surgery of 25% of the baseline amplitude had a sensitivity of 89% and specificity of 82% for the prediction of postoperative BBD aggravation.
In spinal surgery, postoperative bowel bladder disorder (BBD) occurs at a relatively high rate in patients with conditions such as spina bifida, spinal cord tumor, and spinal tethered cord syndrome. Intraoperative monitoring of the external anal sphincter (EAS) for the prevention of intraoperative neurological deficit was first reported by James et al. [1], and subsequently, there have been many reports on monitoring with transcranial muscle action potentials (Tc-MsEP), including that of the EAS, in spinal surgery [2-16].
We have previously described plug-surface electrodes for the intraoperative monitoring of the EAS, which has a lower derivation rate than other muscles [2]. Based on the possible influence of the electrode type on the derivation rate, we subsequently used needle electrodes for monitoring the EAS with Tc-MsEP. In the current study, we compared the rates of postoperative BBD after intraoperative spinal cord monitoring using needle and plug-surface electrodes and examined the appropriate alarm points for waveform deterioration in EAS monitoring.
At our hospital, 269 consecutive spinal surgeries with intraoperative neurophysiological monitoring with Tc-MsEP were performed from April 2009 to December 2011. This study was approved by the Institutional Review Board of Nagoya University Hospital (IRB approval no., 354-3) and each patient provided informed consent before enrollment. After the exclusion of 25 reoperation cases and five dialysis patients (preoperative motor status itself was not considered in the exclusion criteria), 239 patients (104 males and 135 females) were included in the study. The mean age of the patients at surgery was 48.5 years (range, 8.86 years). There were 59 cervical cases (24.7%), 107 thoracic cases (44.8%), and 73 lumbar cases (30.5%); 23 cases (9.6%) had preoperative BBD. Bladder bowel function was measured using the Japanese Orthopaedic Association (JOA) score before and after the surgery, with a JOA score of <0 indicating preoperative BBD and a decline in a score of >1 indicating postoperative BBD aggravation. Among the 239 patients, plug-surface electrodes were used in 123 consecutive patients and needle electrodes were used in 116 consecutive patients for monitoring the EAS with Tc-MsEP (Fig. 1). Details of the two groups are shown in Table 1. Waveform derivation, postoperative BBD aggravation, and sensitivity and specificity for the prediction of BBD aggravation at an amplitude reduction of 70% were investigated. The cutoff for the amplitude reduction with needle electrodes for the prediction of postoperative BBD aggravation was also examined.
A minimal benzodiazepine dose was used as a pre-anesthetic to avoid possible suppression of waveform latency and amplitude. Propofol (3.4 mg/kg), fentanyl (2 mg/kg), and vecuronium (0.12.0.16 mg/kg) were administered for induction, and anesthesia was maintained with propofol (50.100 μg/kg/min), fentanyl (1.2.5 μg/kg/hr), and vecuronium (0.01.0.04 mg/kg/hr). Concomitant hypotensive anesthesia was induced as appropriate with continuous prostaglandin E1 and a short-acting β1 blocker (landiolol). Patients were maintained in a normothermic state and their temperature was raised in the event of possible intraoperative spinal damage. End-tidal CO2 was maintained in the reference range throughout the surgery. For intraoperative body temperature monitoring, a catheter with a vesical temperature sensor was used. Hemodynamic data were electronically recorded with invasive arterial blood pressure (BP) monitoring. Systolic BP variation was measured during the surgery, and systolic BP was determined at the time of waveform deterioration.
An MS120B (Nihon-Kohden, Tokyo, Japan) was used to perform transcranial stimulation, with parameters of 5 stimuli in a row at 2-ms intervals, a constant biphasic current of 200 mA for 500 μs, a 50–1000-Hz filter, and a 100-ms epoch time with ≤20 recorded signal responses. The stimulated point was 2 cm anterior and 6 cm lateral from the Cz location over the cerebral cortex motor area. Using a Neuromaster MEE-1232 ver. 05.10 (Nihon-Kohden), which is expandable to 32 channels, muscle action potentials were recorded from the upper and lower extremities via a pair of needle electrodes that were 3–5 Tc-MsEPs apart. The bilateral trapezius, triceps, deltoid, biceps, brachioradialis, abductor digit minimi, extensor carpi ulnaris, adductor longus, quadriceps femoris, hamstrings, tibialis anterior, gastrocnemius, and abductor halluces were used as target muscles. From April 2009 to August 2010, we used 2-channel plug-surface electrodes for the EAS for consecutive patients, and from September 2010 to December 2011, we used bilateral pairs of needle electrodes that were 2 cm apart outside the anus for consecutive patients (Fig. 1). Tc-MsEP data from these muscles were used for analysis.
Significance was assessed using either the Student t-test or the Fisher’s exact test. Receiver operating characteristic (ROC) curves were used to determine the cutoff amplitude. Sensitivity and specificity at the optimal cutoff values were calculated. All p<0.05 were considered significant in all analyses. Data analysis was performed with IBM SPSS ver. 22.0 for Windows (IBM Corp., Armonk, NY, USA).
The waveform derivation rate was significantly higher using needle electrodes (91.3% [106/116] versus 76.4% [94/123], p<0.01) (Fig. 2). This rate was also significantly higher using needle electrodes in cases with preoperative BBD (60% [6/10] versus 16% [2/13], p<0.05) (Fig. 3A) and without preoperative BBD (94.3% [100/106] versus 83.6% [92/110], p<0.05] (Fig. 3B). The etiology of postoperative BBD is shown for cases treated using needle and plugsurface electrodes in Table 2. The incidence of postoperative BBD aggravation was 7.1% (17/239) in all cases, 6.9% (8/116) using needle electrodes, and 7.3% (9/123) using plug-surface electrodes (Table 2).
In patients with baseline waveform detection wherein needle electrodes were used, the sensitivity and specificity for the prediction of BBD aggravation were 88% and 85%, respectively (Table 3). Using plug-surface electrodes, the sensitivity and specificity for the prediction of BBD aggravation were approximately 89% and 80%, respectively (Table 4). When using needle electrodes for the EAS, the intraoperative Tc-MsEP cutoff amplitude at the end of surgery in the ROC analysis was 25% of the baseline amplitude, with a sensitivity of 89% and specificity of 82% for the prediction of BBD aggravation (Fig. 4). There were no complications with plug-surface or needle electrodes.
In 1953, Floyd and Walls [3] first reported an electromyogram using a surface electrode placed on the skin of the anus. Monitoring of the EAS with Tc-MsEPs is now widely performed in spinal surgery to prevent intraoperative neurological deficit [1,4,7-14,16,17]; in addition, electrophysiological tests for bladder and rectal function have been developed. Many institutions now use monitoring of the EAS with Tc-MsEP to prevent intraoperative paralysis during spinal surgery. The EAS has pudendal innervation, in which the dominant area is S2–4 and is thus useful for intraoperative monitoring of urinary function and dysuria due to spinal diseases [6].
The plug-surface electrode is relatively simple and less invasive, but the waveform derivation rate is low [7]. In our series, the waveform derivation rate from the EAS using plug-surface electrodes was 76.4%, which was significantly lower than the rate of 91.3% using needle electrodes. The sensitivity and specificity for the prediction of BBD aggravation based on a waveform reduction of ≥70% in monitoring of the EAS with Tc-MsEP were similar for the two electrodes. However, we found that it was difficult to detect baseline waveform using a plug-surface electrode in a case with preoperative BBD; thus, it is preferable to use needle electrodes for the anus. In fact, we have always used needle electrodes for all extremities.
Muramoto et al. [8,13] reported cutoff values for lower limb muscles concerning paralysis exacerbation in spinal thoracic surgery, but there have been no reports on cutoff values for intraoperative monitoring with regard to postoperative BBD. In our study, ROC analysis using needle electrodes for monitoring of the EAS with Tc-MsEP gave a cutoff of 25% of the baseline amplitude for the prediction of postoperative BBD aggravation. A waveform amplitude reduction of 70% in spinal surgery is the recommended criterion for an alarm point [12], and an alarm point of 70% waveform reduction may also be appropriate for the EAS. However, there are many false-positive cases at the 70% alarm point, and further study is needed to improve the accuracy of the monitoring. Specifically, there is a need to evaluate the absolute amplitude and to consider both single-train and double-train stimulation methods.
In patients with baseline waveform detection, the sensitivity and specificity for the prediction of BBD aggravation did not differ significantly between the two electrodes. However, deeper muscles could be detected using needle electrodes, and this resulted in a better waveform derivation rate and no complications. Therefore, we consider needle electrodes to be the most useful for monitoring BBD, although this may be difficult in patients with injuries that might be caused by needle insertion, as well as in patients with hemorrhoids.
In patients with baseline waveform detection, sensitivity and specificity were equivalent for needle and plugsurface electrodes; however, the waveform derivation rate was significantly higher with needle electrodes. The study provides the first report of a cutoff value of 25% for the prediction of postoperative BBD aggravation using needle electrodes and shows that needle electrodes are effective for monitoring the EAS muscles during spinal surgery, particularly in cases with preoperative BBD.
Conflict of Interest
No potential conflict of interest relevant to this article was reported. Funding was from institutional sources only.
Notes
Author Contributions
Kobayashi K analyzed data and wrote the manuscript; Ando K, Yagi Y, Ito K, Tsushima M, Morozumi M, Tanaka S, Machino M, Ota K, Matsuyama Y, Ishiguro N, and Imagama S designed the study and collected data.
Fig. 1.
Two types of electrodes used for the external anal sphincter. (A) Needle electrode. (B) Plug-surface electrode.
Fig. 2.
The waveform derivation rate was significantly higher using the needle electrode (91.3% [106/116] vs. 76.4% [94/123]). *p<0.01.
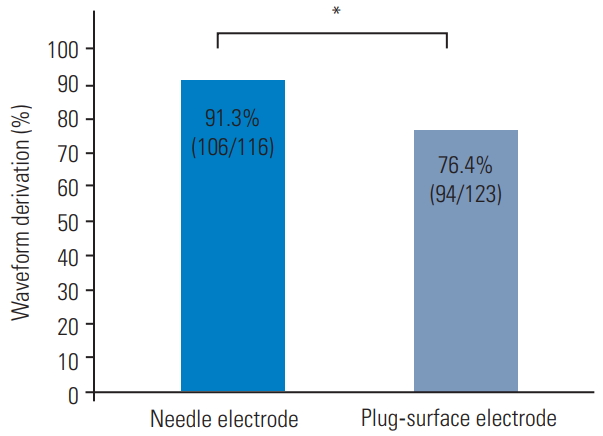
Fig. 3.
(A) In a case with preoperative bowel bladder disorder (BBD), the waveform derivation rate was significantly higher using the needle electrode (60% [6/10] vs. 16% [2/13]). *p<0.05. (B) In a case without preoperative BBD, the waveform derivation rate was also significantly higher using the needle electrode (94.3% [100/106] vs. 83.6% [92/110]). *p<0.05.
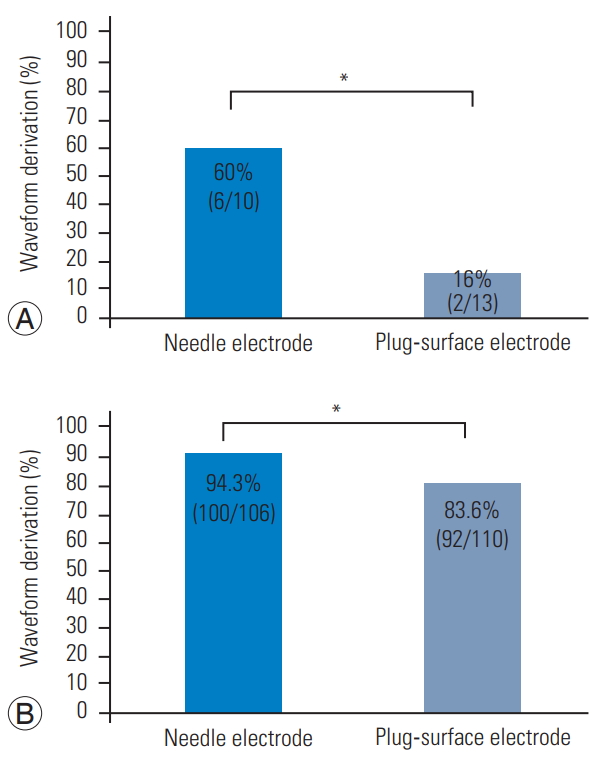
Fig. 4.
Receiver operating characteristic curve for the determination of the cutoff for the prediction of postoperative bowel bladder disorder aggravation using the intraoperative transcranial muscle action potentials amplitude (expressed as % of baseline) obtained for the external anal sphincter using needle electrodes.
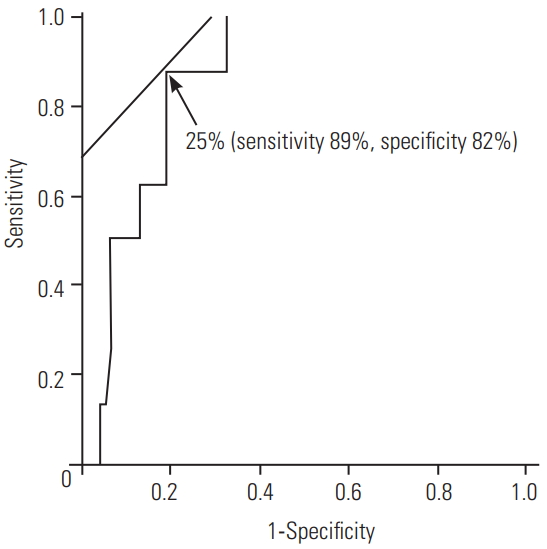
Table 1.
Characteristics of cases treated using monitoring with needle and plug-surface electrodes
Table 2.
Incidence of postoperative bowel bladder disorder aggravation in cases treated using monitoring with needle and plug-surface electrodes
Table 3.
Relationship of postoperative BBD with waveform deterioration with needle electrode
| Variable |
Postoperative BBD |
||
|---|---|---|---|
| Present | Absent | Total | |
| Waveform deterioration (+) | 7 | 15 | 22 |
| Waveform deterioration (−) | 1 | 83 | 84 |
| Total | 8 | 98 | 106 |
References
1. James HE, Mulcahy JJ, Walsh JW, Kaplan GW. Use of anal sphincter electromyography during operations on the conus medullaris and sacral nerve roots. Neurosurgery 1979 4:521–3.


2. Kobayashi K, Ando K, Yagi H, et al. Prevention and prediction of postoperative bowel bladder disorder using an anal plug electrode with Tc-MsEP monitoring during spine surgery. Nagoya J Med Sci 2017 79:459–66.


3. Floyd WF, Walls EW. Electromyography of the sphincter ani externus in man. J Physiol 1953 122:599–609.



4. Haghighi SS, Zhang R. Activation of the external anal and urethral sphincter muscles by repetitive transcranial cortical stimulation during spine surgery. J Clin Monit Comput 2004 18:1–5.


5. Wiesner A, Jost WH. EMG of the external anal sphincter: needle is superior to surface electrode. Dis Colon Rectum 2000 43:116–8.


6. Kumar GS, Rajshekhar V, Babu KS. Intraoperative mapping of sacral nervous system (S2-4). Br J Neurosurg 2006 20:396–402.


7. Inoue S, Kawaguchi M, Takashi S, et al. Intraoperative monitoring of myogenic motor-evoked potentials from the external anal sphincter muscle to transcranial electrical stimulation. Spine (Phila Pa 1976) 2002 27:E454–9.


8. Muramoto A, Imagama S, Ito Z, et al. The cutoff amplitude of transcranial motor-evoked potentials for predicting postoperative motor deficits in thoracic spine surgery. Spine (Phila Pa 1976) 2013 38:E21–7.


9. Ito Z, Matsuyama Y, Ando M, et al. What is the best multimodality combination for intraoperative spinal cord monitoring of motor function?: a multicenter study by the Monitoring Committee of the Japanese Society for Spine Surgery and Related Research. Global Spine J 2016 6:234–41.


10. Teramoto T. Functional characteristics features in anatomy and physiology of the anal canal. Nichisyougekaishi 1990 23:2147–50.

11. Kothbauer KF, Deletis V. Intraoperative neurophysiology of the conus medullaris and cauda equina. Childs Nerv Syst 2010 26:247–53.


12. Ito Z, Imagama S, Sakai Y, et al. A new criterion for the alarm point for compound muscle action potentials. J Neurosurg Spine 2012 17:348–56.


13. Muramoto A, Imagama S, Ito Z, et al. The cutoff amplitude of transcranial motor evoked potentials for transient postoperative motor deficits in intramedullary spinal cord tumor surgery. Spine (Phila Pa 1976) 2014 39:E1086–94.


14. Kobayashi K, Imagama S, Ito Z, et al. Transcranial motor evoked potential waveform changes in corrective fusion for adolescent idiopathic scoliosis. J Neurosurg Pediatr 2017 19:108–15.


15. Nandedkar SD, Sanders DB, Stalberg EV, Andreassen S. Simulation of concentric needle EMG motor unit action potentials. Muscle Nerve 1988 11:151–9.


- TOOLS






