|
 |
- Search
| Asian Spine J > Volume 11(6); 2017 > Article |
|
Abstract
Purpose
The aim of this study was to evaluate the effect of atorvastatin on sensory and motor function in patients with acute spinal cord injury.
Overview of Literature
The prevalence and incidence of traumatic spinal cord injury are increasing. Statins are well established for use in hypercholesterolemia as well as during anti-inflammatory events.
Methods
This clinical trial study included 60 patients with acute spinal cord injury. These were randomly divided into two groups: the case group which received atorvastatin and also underwent surgical therapy and the control group which only underwent surgical therapy.
Results
The severity of spinal cord lesions was evaluated based on the Frankel grade at three periods; this showed no significant difference between the two groups. Comparisons of the levels of pain between the groups based on a Visual Analog Scale system showed no significant difference at the three periods.
Conclusions
We observed no improvement at the 3- and 6-month follow-up in patients who were administered atorvastatin. However, a comparison of the two groups based on pain severity demonstrated a significant difference, suggesting that atorvastatin had a positive effect on patients with spinal cord injury.
The prevalence and incidence of traumatic spinal cord injury (SCI) have been increasing. SCI has an early and a secondary phase. The early phase occurs because of physical injury to the spinal cord and causes devastation of the local vascular system, leading to microhemorrhaging of the white and gray matter [1]. The secondary phase results from a severe inflammation response, vascular changes, glutamate excitotoxicity, ischemia reperfusion injury, ionic hemostasis changes, and oxidative cell injury [23]. The term ãcomplete injuryã indicates no sensory and/or motor function more than three segments below the injury level, whereas ãincomplete injuryã indicates the existence of some sensory and motor function more than three segments below the injury level [45]. Many strategies using neuroprotective agents have demonstrated low success in human clinical trials [6].
The use of statins for hypercholesterolemia is well established; they inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase, which converts HMG-CoA to mevalonate [78]. They also have an anti-inflammatory role [9], and they provide neuroprotective effects by reducing the levels of inflammatory factors such as tumor necrosis factor öÝ (TNF-öÝ), and interleukin 1öý (IL-1öý) [1011]. They exhibit antioxidant effects and reduce endothelial nitric oxide, which causes neuronal degeneration in the secondary phase of SCI [12]. Statins prescribed for hypercholesterolemia include atorvastatin, lovastatin, and pravastatin [13]. Pannu et al. [14] demonstrated that using atorvastatin for 1 week in patients with SCI resulted in a significant reduction in the inflammatory condition as well as neuronal recovery, increased motor function, and reduced neuronal cell death. Another study by Pannu et al. [15] reported significant neuroprotective effects of statins in post-SCI treatment. Conversely, Pathak et al. [16] demonstrated anti-oxidative and anti-analgesic effects of atorvastatin in the spinal cord. The aim of the present study was to evaluate the effect of atorvastatin on sensory and motor function in patients with acute SCI.
This was a clinical trial in which all subjects were patients with acute SCI referred to the education center of Imam Reza hospital of Tabriz university of Medical Sciences during 2014ã2015. This study was registered at the Iranian Registration of Clinical Trial Center (IRCT): 2014032113947. It included 60 patients with acute SCI at Frankel grades AãC. The patients were matched by sex, body weight, and Frankel grade and were randomly divided into two groups (n=30 patients each). The case group received atorvastatin immediately on admission in addition to surgical therapy, whereas the control group underwent only surgical therapy. The following inclusion criteria were applicable: patients with acute SCI aged 18ã70 years, with Frankel grade classes AãC and fracture of the vertebrae C4ãL2. The exclusion criteria were as follows: renal or hepatic disease, penetration injury, traumatic brain injury, lactation or pregnancy, recent alcohol consumption, neurologic or psychological disease, life-threatening injuries, and the inability for oral drug intake. Severity of spinal lesions was determined according to the Frankel grade, a classification system that grades patients from A to E on the basis of their sensory and motor lesions as follows: A, no sensation or motion; B, no motion but a little sensation remains; C, sensation is good and there is some motion, although it is not usable; D, sensation is good and although motion is incomplete, it is usable; and E, natural sensation and motion. During the study, the case group received atorvastatin at a dosage of 5 mg/kg orally daily for 4 weeks. The patients were evaluated for sensory and motor function and the level of pain on admission and 3 and 6 months later.
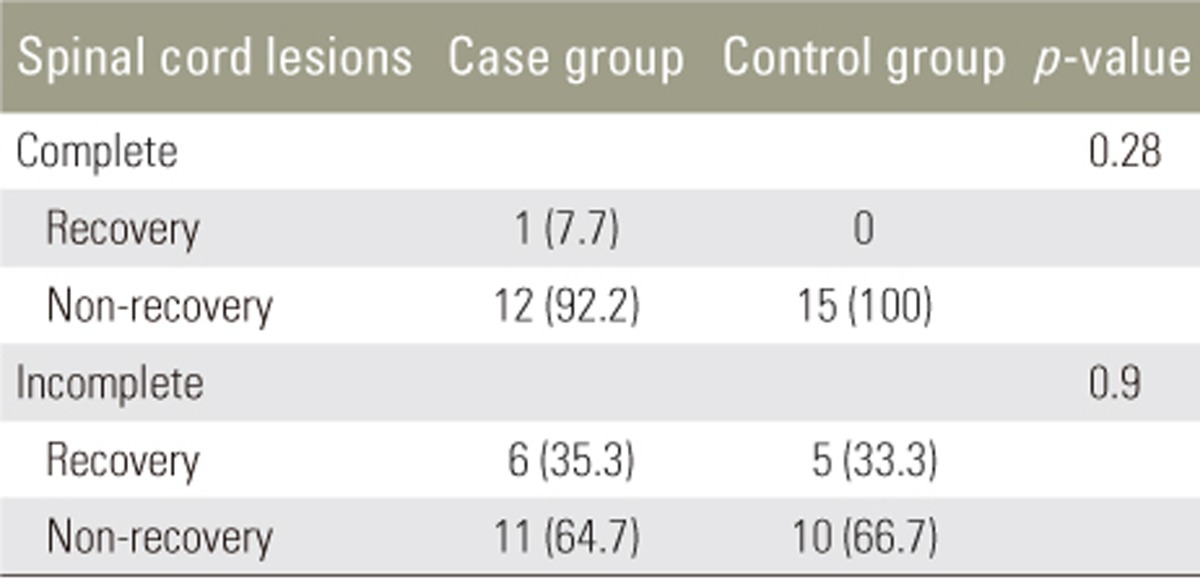
Different methods have been proposed for evaluating postoperative pain, including the numerical rating scale, single descriptive scale, visual analog scale (VAS), and University of Melbourne pain scale. VAS is the most common method used in studies determining pain according to behavioral factors [17]. The capability and adequacy of this method for evaluating postoperative pain have been demonstrated [1819], and it was chosen to evaluate the levels of pain in the present study. The patients' recovery or non-recovery status were also evaluated based on neurologic symptoms. Recovery was considered as achieving full sensory and motor function.
The study design was approved by the ethics committee of Tabriz University of Medical Science. Prior to the study, all aspects of the study were explained to the patients, who gave their informed consent. The patients' information was kept confidential.
Data are presented as meanôÝstandard deviation. The analysis used SPSS ver. 16 statistical software (SPSS Inc., Chicago, IL, USA) and included descriptive statistics, calculations of abundance and percentages, the T test to compare quantitative variables for independent groups, and the chi-square test for qualitative variables. A p-value <0.05 was considered statistically significant.
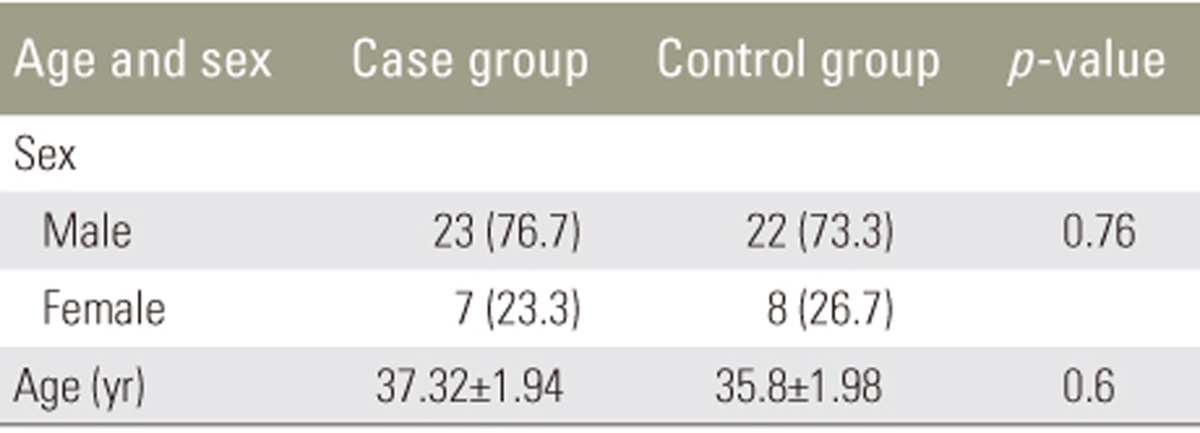
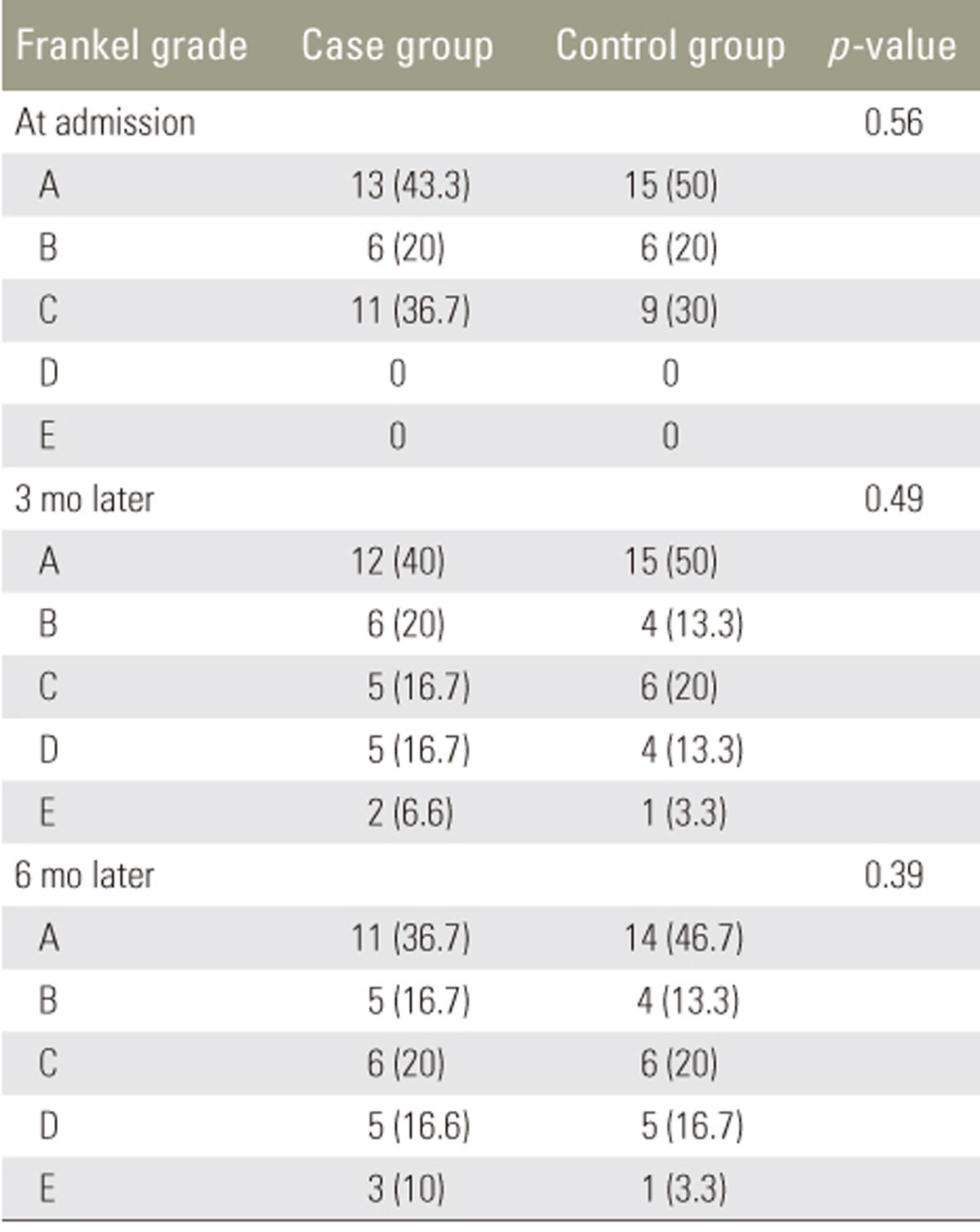
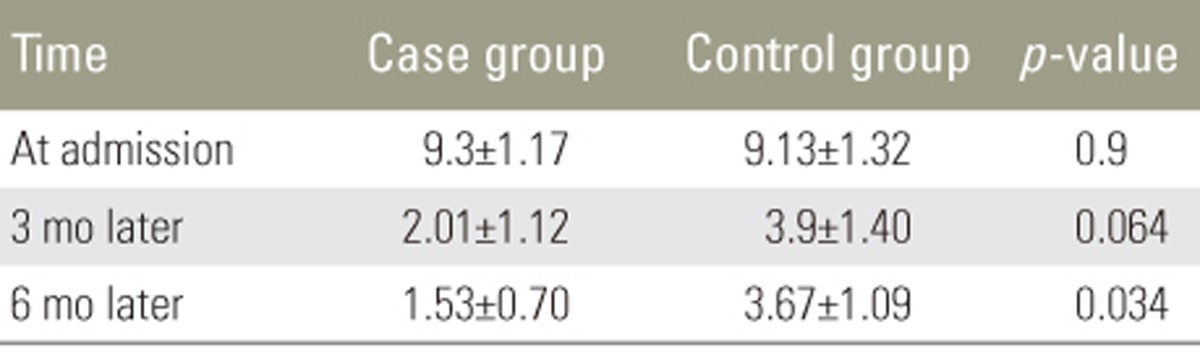
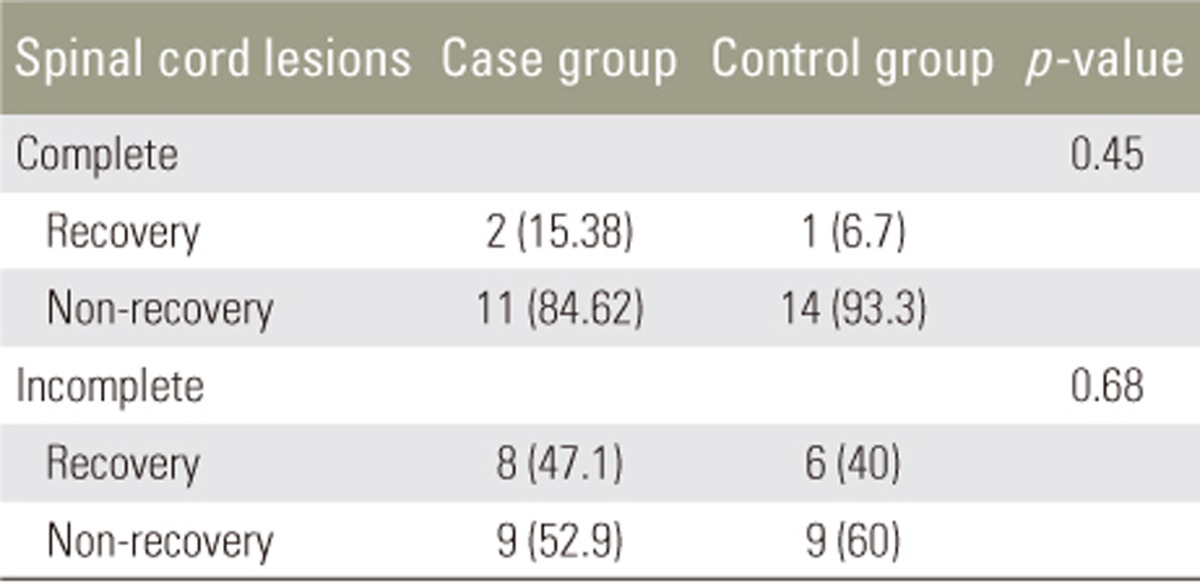
All 60 patients (30 in each group) completed the study. In the case group, 23 patients (77%) were men and 7 (23%) were women; the mean age was 37ôÝ1.9 years (range, 22ã54 years). In the control group, 22 (73%) were men and 8 (27%) were women, and the mean age was 35.8ôÝ1.98 years (range, 22ã54 years). The groups were matched for sex and age at the beginning of the study (Table 1). Severity of spinal cord lesions was evaluated at three periods based on the Frankel grading system (Table 2). At the 3- and 6-month follow-ups, neither group showed significant sensory and motor improvement. Table 3 shows a comparison of the levels of pain of the patients between the two groups based on the VAS system; this showed that atorvastatin had no significant effect on the level of pain at the early 3-month follow-up, but at the 6-month follow-up, atorvastatin reduced pain in the case group (p=0.034).
We observed no improvement in the 3- and 6-month follow-up in the patients who received atorvastatin. However, the comparison of pain severity in the two groups demonstrated a significant difference at 6 months, suggesting that atorvastatin has a positive effect on pain improvement in patients with SCI over that period. Acute SCI is one of the most common disorders in the field of neurosurgery, often resulting from driving and occupational accidents, falling, and natural disasters, and it results in a large number of patients being referred to trauma centers [20]. SCI involves two stages. The first stage occurs because of primary mechanical trauma and the second stage results from inflammation, apoptosis of cells, and oxidative stress [21]. High levels of nitric oxide reduce the mitochondrial function and increase neuronal death [2223]. It has been shown that statins can increase the level of endothelial nitric oxide synthase and reduce the level of inducible nitric oxide, leading to a decrease of stroke in animal models [2425]. Statins are known for their role in the reduction of cholesterol, but they also have important antiinflammatory, neuroprotective, and antioxidant effects [26]. Statins have limited side effects, such as rare cases of neuropathy and an incidence of myopathy of 1%ã5% [27]; thus, they can be considered safe for patients. After SCI, there is an increase in the levels of TNF-öÝ, IL-1öý, and other cytokines, leading to inflammation [28]. Therefore, we evaluated the effect of atorvastatin on neuroprotection and pain relief in patients with acute SCI who are suffering from sensory and motor dysfunction. However, we found no significant improvement in the case group based on the Frankel classification. However, the improvement was more remarkable after 3 and 6 months. The lack of a significant improvement with atorvastatin was in contrast with the results of a previous study [15]. Pathak et al. [16] demonstrated that atorvastatin reduced pain at a dose of 10 mg/kg to a greater extent than it did at doses of 3 and 30 mg/kg; however, the range of usage across other studies has been 0.5ã30 mg/kg [2930]. The comparison in the level of pain, based on the VAS system, over two periods postoperatively is shown in Table 3. The level of VAS pain at the beginning of study in the case group was 9.3ôÝ1.17 and that in the control group was 9.13ôÝ1.32. There was no significant difference between the two groups at the 3-month follow-up (p=0.063), but at the 6-month follow-up there was a significant reduction in the level of pain in the case group (p=0.043). Thus, it seems that atorvastatin has positive effect at a dose of 5 mg/kg in patients with acute SCI.
In future, we recommend further studies with more cases and the use of atorvastatin in different doses over a continuous period. Increasing the number of case group patients would be important; the number of patients in the present study was limited.
This study demonstrated that atorvastatin had no significant effect on motor and sensory improvement over the 3- and 6-month follow-up in patients with SCI. However, it could reduce the levels of pain at 6 months in these patients. The investigation of the recovered or non-recovered status of the patients demonstrated no significant effect of atorvastatin in the case group.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
1. Wilson JR, Forgione N, Fehlings MG. Emerging therapies for acute traumatic spinal cord injury. CMAJ 2013;185:485ã492. PMID: 23228995.



2. Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res 2007;161:217ã233. PMID: 17618980.


3. Rowland JW, Hawryluk GW, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 2008;25:E2.

4. American Spinal Cord Injury Association. Standards for neurological classification of spinal cord injury patients. Richmond: American Spinal Cord Injury Association; 1990.
5. Waters RL, Adkins RH, Yakura JS. Definition of complete spinal cord injury. Paraplegia 1991;29:573ã581. PMID: 1787981.


6. Tator CH, Hashimoto R, Raich A, et al. Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. J Neurosurg Spine 2012;17:157ã229. PMID: 22985382.


7. Andrews TC, Ballantyne CM, Hsia JA, Kramer JH. Achieving and maintaining National Cholesterol Education Program low-density lipoprotein cholesterol goals with five statins. Am J Med 2001;111:185ã191. PMID: 11530028.


8. Schachter M. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005;19:117ã125. PMID: 15660968.


9. Weitz-Schmidt G. Statins as anti-inflammatory agents. Trends Pharmacol Sci 2002;23:482ã486. PMID: 12368073.


10. Holmberg E, Nordstrom T, Gross M, Kluge B, Zhang SX, Doolen S. Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma 2006;23:1366ã1378. PMID: 16958588.


11. Mann CM, Lee JH, Hillyer J, Stammers AM, Tetzlaff W, Kwon BK. Lack of robust neurologic benefits with simvastatin or atorvastatin treatment after acute thoracic spinal cord contusion injury. Exp Neurol 2010;221:285ã295. PMID: 19931252.


12. Cimino M, Gelosa P, Gianella A, Nobili E, Tremoli E, Sironi L. Statins: multiple mechanisms of action in the ischemic brain. Neuroscientist 2007;13:208ã213. PMID: 17519364.


13. Stuve O, Youssef S, Steinman L, Zamvil SS. Statins as potential therapeutic agents in neuroinflammatory disorders. Curr Opin Neurol 2003;16:393ã401. PMID: 12858078.


14. Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res 2005;79:340ã350. PMID: 15605375.


15. Pannu R, Christie DK, Barbosa E, Singh I, Singh AK. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J Neurochem 2007;101:182ã200. PMID: 17217414.


16. Pathak NN, Balaganur V, Lingaraju MC, et al. Atorvastatin attenuates neuropathic pain in rat neuropathy model by down-regulating oxidative damage at peripheral, spinal and supraspinal levels. Neurochem Int 2014;68:1ã9. PMID: 24513038.


17. Firth AM, Haldane SL. Development of a scale to evaluate postoperative pain in dogs. J Am Vet Med Assoc 1999;214:651ã659. PMID: 10088012.


18. Impellizeri JA, Tetrick MA, Muir P. Effect of weight reduction on clinical signs of lameness in dogs with hip osteoarthritis. J Am Vet Med Assoc 2000;216:1089ã1091. PMID: 10754668.


19. Conzemius MG, Hill CM, Sammarco JL, Perkowski SZ. Correlation between subjective and objective measures used to determine severity of postoperative pain in dogs. J Am Vet Med Assoc 1997;210:1619ã1622. PMID: 9170089.


20. Wilson JR, Cho N, Fehlings MG,Acute traumatic spinal cord injury: epidemiology, evaluation, and management. Patel V, Patel A, Harrop J, Burger E, editors. Spine surgery basics. Berlin: Springer; 2014. p.399ã409.
21. Keane RW, Davis AR, Dietrich WD. Inflammatory and apoptotic signaling after spinal cord injury. J Neurotrauma 2006;23:335ã344. PMID: 16629620.


22. Bal-Price A, Brown GC. Inflammatory neurodegeneration mediated by nitric oxide from activated glia-inhibiting neuronal respiration, causing glutamate release and excitotoxicity. J Neurosci 2001;21:6480ã6491. PMID: 11517237.



23. Leist M, Fava E, Montecucco C, Nicotera P. Peroxynitrite and nitric oxide donors induce neuronal apoptosis by eliciting autocrine excitotoxicity. Eur J Neurosci 1997;9:1488ã1498. PMID: 9240406.


24. Yamada M, Huang Z, Dalkara T, et al. Endothelial nitric oxide synthase-dependent cerebral blood flow augmentation by L-arginine after chronic statin treatment. J Cereb Blood Flow Metab 2000;20:709ã717. PMID: 10779015.


25. Pahan K, Sheikh FG, Namboodiri AM, Singh I. Lovastatin and phenylacetate inhibit the induction of nitric oxide synthase and cytokines in rat primary astrocytes, microglia, and macrophages. J Clin Invest 1997;100:2671ã2679. PMID: 9389730.



26. Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005;45:89ã118. PMID: 15822172.



27. Ziajka PE, Wehmeier T. Peripheral neuropathy and lipid-lowering therapy. South Med J 1998;91:667ã668. PMID: 9671841.


28. Wang CX, Olschowka JA, Wrathall JR. Increase of interleukin-1beta mRNA and protein in the spinal cord following experimental traumatic injury in the rat. Brain Res 1997;759:190ã196. PMID: 9221936.


29. Yang D, Han Y, Zhang J, Chopp M, Seyfried DM. Statins enhance expression of growth factors and activate the PI3K/Akt-mediated signaling pathway after experimental intracerebral hemorrhage. World J Neurosci 2012;2:74ã80. PMID: 23482588.



30. Stepien KM, Tomaszewski M, Luszczki JJ, Czuczwar SJ. The interactions of atorvastatin and fluvastatin with carbamazepine, phenytoin and valproate in the mouse maximal electroshock seizure model. Eur J Pharmacol 2012;674:20ã26. PMID: 22056838.


- TOOLS
-
METRICS

- Related articles in ASJ
-
Optogenetics Applications for Treating Spinal Cord Injury2015 April;9(2)