1. Ross PD, Fujiwara S, Huang C, et al. Vertebral fracture prevalence in women in Hiroshima compared to Caucasians or Japanese in the US. Int J Epidemiol 1995 24:1171–7.


2. Tsujio T, Nakamura H, Terai H, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine (Phila Pa 1976) 2011 36:1229–35.

3. Takahashi S, Hoshino M, Takayama K, et al. Predicting delayed union in osteoporotic vertebral fractures with consecutive magnetic resonance imaging in the acute phase: a multicenter cohort study. Osteoporos Int 2016 27:3567–75.


4. Muratore M, Ferrera A, Masse A, Bistolfi A. Osteoporotic vertebral fractures: predictive factors for conservative treatment failure: a systematic review. Eur Spine J 2018 27:2565–76.


5. Goldstein S, Smorgick Y, Mirovsky Y, Anekstein Y, Blecher R, Tal S. Clinical and radiological factors affecting progressive collapse of acute osteoporotic compression spinal fractures. J Clin Neurosci 2016 31:122–6.


6. Ha KY, Kim YH. Risk factors affecting progressive collapse of acute osteoporotic spinal fractures. Osteoporos Int 2013 24:1207–13.


7. Kanchiku T, Imajo Y, Suzuki H, Yoshida Y, Taguchi T. Usefulness of an early MRI-based classification system for predicting vertebral collapse and pseudoarthrosis after osteoporotic vertebral fractures. J Spinal Disord Tech 2014 27:E61–5.


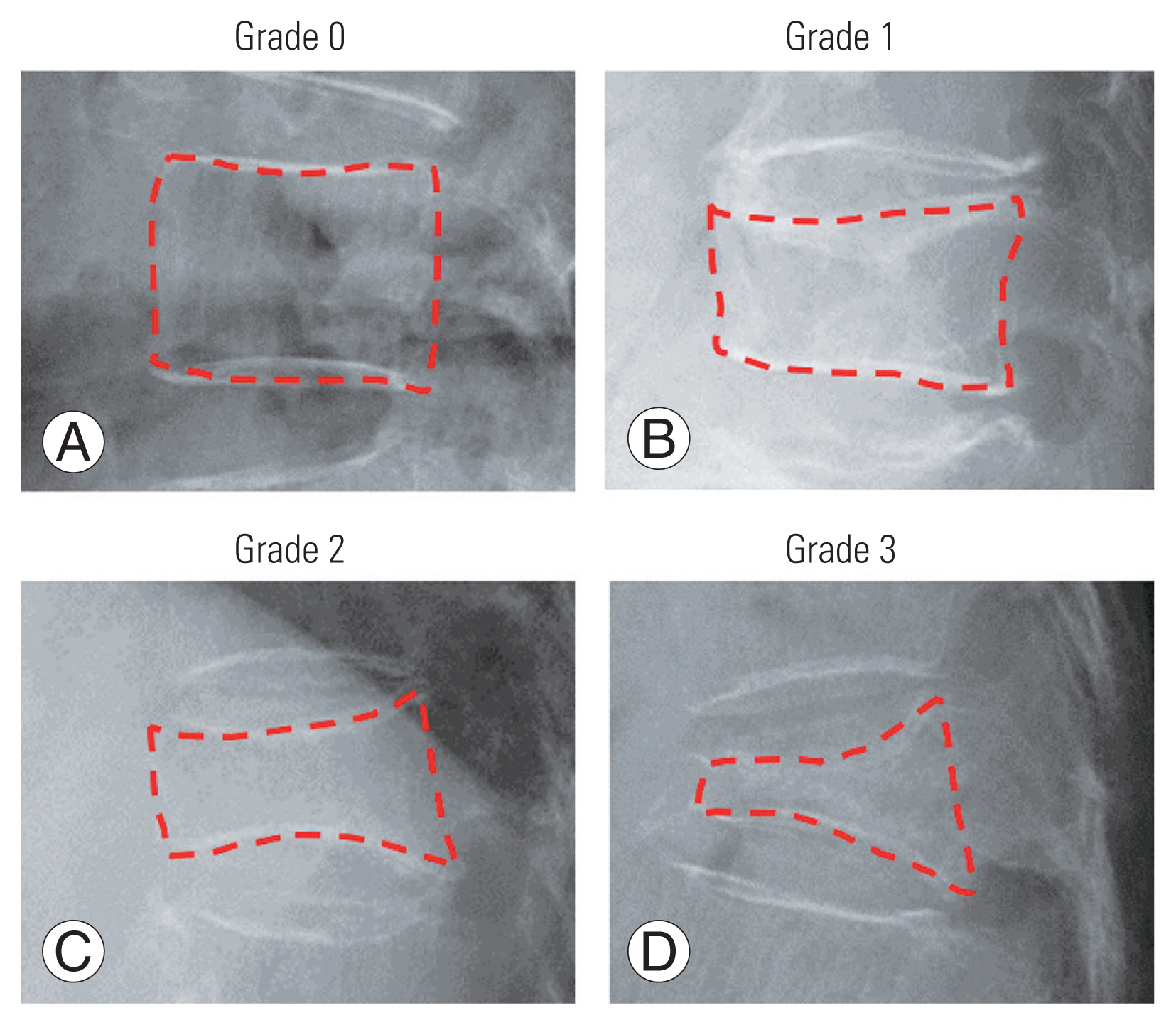
8. Sugita M, Watanabe N, Mikami Y, Hase H, Kubo T. Classification of vertebral compression fractures in the osteoporotic spine. J Spinal Disord Tech 2005 18:376–81.


9. Sennerby U, Melhus H, Gedeborg R, et al. Cardiovascular diseases and risk of hip fracture. JAMA 2009 302:1666–73.


10. Imai Y, Hasegawa K. The Revised Hasegawa’s Dementia Scale (HDS-R): evaluation of its usefulness as a screening test for dementia. Hong Kong J Psychiatry 1994 4:20.
11. Sherry J, Sherry M, Tauferner S. Rehabilitation guidelines for lumbar spondylolysis/spondylolisthesis. Madison (WI): UW Health Sports Rehabilitation; 2017.
12. Yoshioka T, Morio Y, Nagashima H, Yamasaki D, Yamamoto K. Usefulness of MRI for predicting collapsing rate of fractured vertebrae. Orthop Traumatol 1999 48:1077–80.

13. Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993 8:1137–48.


14. Genant HK, Jergas M, Palermo L, et al. Comparison of semiquantitative visual and quantitative morphometric assessment of prevalent and incident vertebral fractures in osteoporosis. J Bone Miner Res 1996 11:984–96.


15. Adams JE, Lenchik L, Roux C, Genant HK. Vertebral fracture initiative: part II. radiological assessment of vertebral fracture. Nyon: International Osteoporosis Foundation; 2010.
16. Matsuoka H, Sakano Y. Assessment of cognitive aspect of pain: development, reliability, and validation of Japanese version of Pain Catastrophizing Scale. Jpn J Psychosom Med 2007 47:95–102.
20. Kaukinen PT, Arokoski JP, Huber EO, Luomajoki HA. Intertester and intratester reliability of a movement control test battery for patients with knee osteoarthritis and controls. J Musculoskelet Neuronal Interact 2017 17:197–208.


21. Horiuchi T, Kobayashi Y, Hosoi T, Ishibashi H, Yamamoto S, Yatomi N. The assessment of the reliability and the validity of the EOQOL questionnaire of osteoporotics: QOL assessment of elderly osteoporotics by EOQOL. Nihon Ronen Igakkai Zasshi 2005 42:229–34.


22. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965 14:61–5.
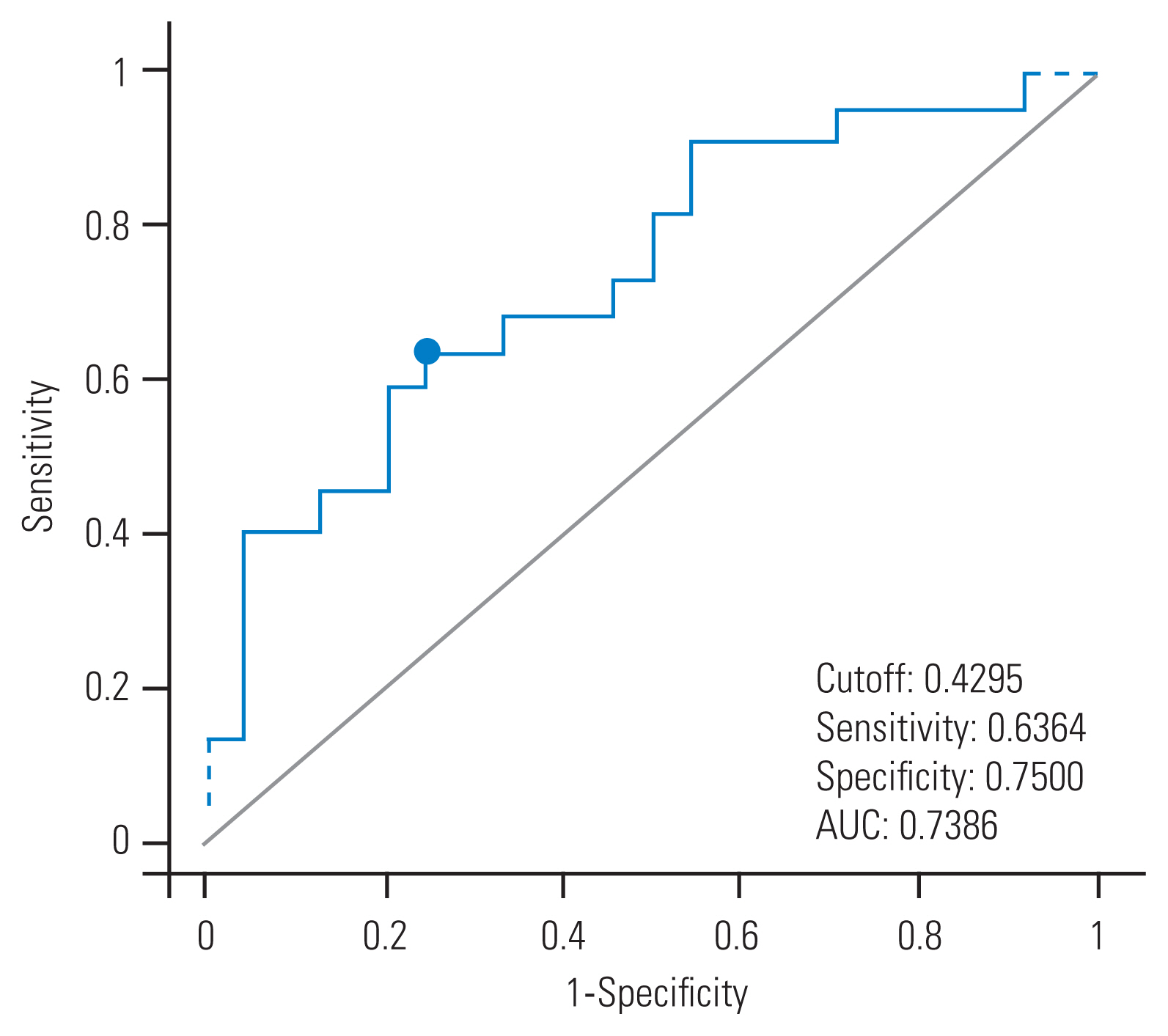
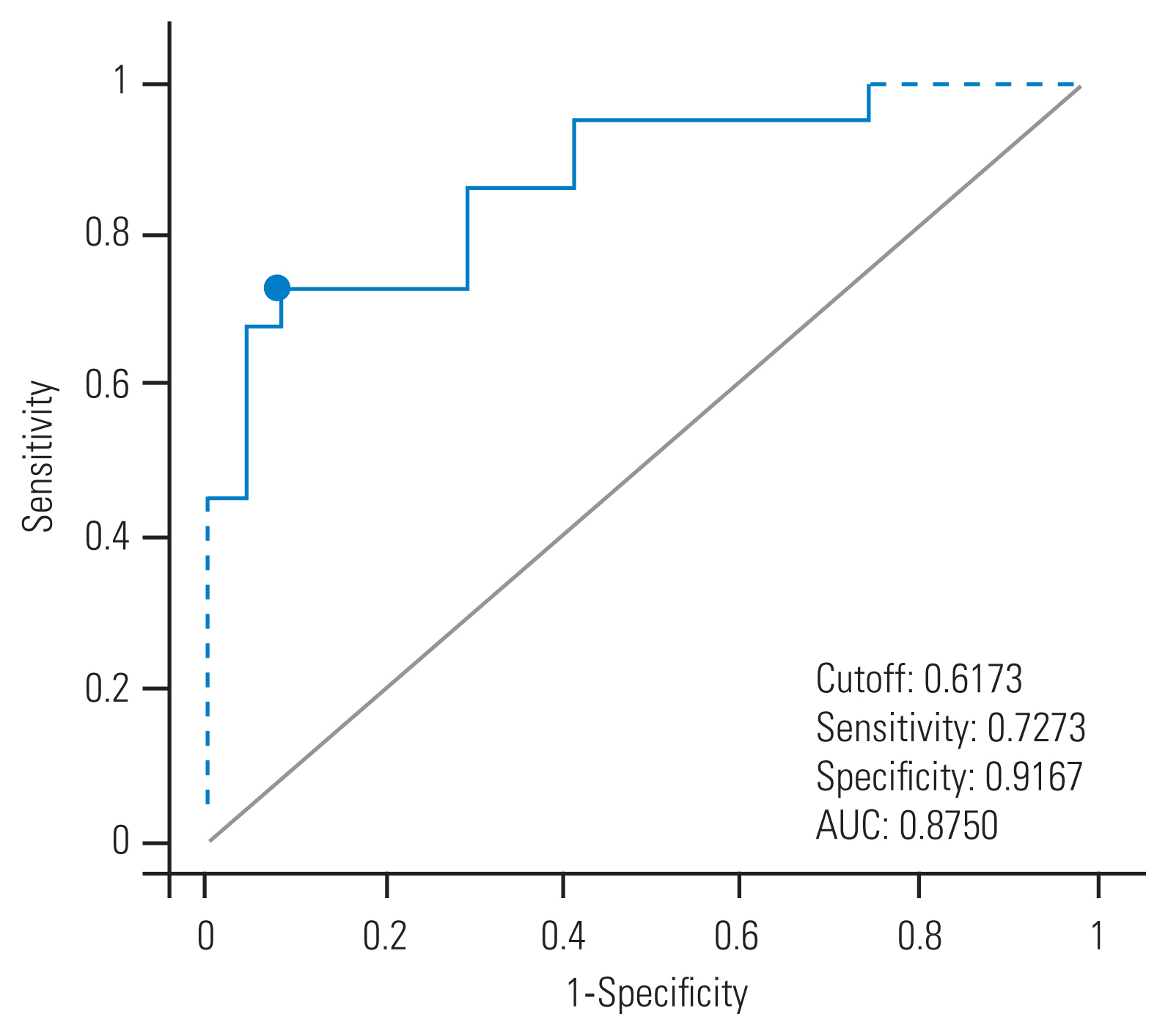
23. Swets JA. Measuring the accuracy of diagnostic systems. Science 1988 240:1285–93.


24. Persy V, D’Haese P. Vascular calcification and bone disease: the calcification paradox. Trends Mol Med 2009 15:405–16.


25. Chen SJ, Lin CS, Lin CL, Kao CH. Osteoporosis is associated with high risk for coronary heart disease: a population-based cohort study. Medicine (Baltimore) 2015 94:e1146.


26. Pennisi P, Signorelli SS, Riccobene S, et al. Low bone density and abnormal bone turnover in patients with atherosclerosis of peripheral vessels. Osteoporos Int 2004 15:389–95.


29. Wertli MM, Eugster R, Held U, Steurer J, Kofmehl R, Weiser S. Catastrophizing: a prognostic factor for outcome in patients with low back pain: a systematic review. Spine J 2014 14:2639–57.


30. Iwata A, Kanayama M, Oha F, Hashimoto T, Iwasaki N. Does spinopelvic alignment affect the union status in thoracolumbar osteoporotic vertebral compression fracture? Eur J Orthop Surg Traumatol 2017 27:87–92.