Comparative Outcome Data Using Different Techniques for Posterior Lumbar Fusion: A Large Single-Center Study
Article information
Abstract
Study Design
Retrospective single-center study.
Purpose
This study aims to evaluate perioperative and intermediate-term clinical outcomes of patients undergoing different lumbar fusion techniques.
Overview of Literature
Various open and minimally invasive techniques for lumbar fusion are available, but previous studies comparing lumbar fusion techniques have heterogeneous data, making interpretation challenging.
Methods
Between 2011 and 2018, data from 447 consecutive patients undergoing one/two-level lumbar fusion were analyzed. Posterior lumbar interbody fusion (PLIF) with bilateral muscle strip or Wiltse approach, open transforaminal lumbar interbody fusion (TLIF) and minimally invasive TLIF, and posterolateral fusion only were among the surgical techniques used. Core outcomes measure index (COMI) questionnaires were distributed before surgery and at 3 months, 1 year, and 2 years postoperatively to establish patient self-reported outcome measures. Demographic data (age, gender, and body mass index [BMI]) for each patient were also collected in addition to surgical indication, previous operative history, perioperative outcomes, and complications, and whether later revision surgery was required. Pearson’s chi-square test, Kruskal-Wallis test, repeated measure mixed-effects models, and ordinal logistic regression were used for statistical analysis.
Results
Postoperative COMI scores improved across all procedures compared with pre-surgery (p<0.001). There was no significant difference between different postoperative COMI scores. Significant predictors of higher postoperative COMI score included higher pretreatment COMI score (p≤0.001), previous surgery (p≤0.04), younger age (p≤0.05), higher BMI (p≤0.005), and the indications of lytic spondylolisthesis (p=0.02) and degenerative disc disease (p<0.001). Patients undergoing minimally invasive TLIF had a significantly shorter post-surgery stay than patients undergoing open PLIF (Kruskal-Wallis test, p=0.03).
Conclusions
At 2 years postoperatively, there was no significant difference in clinical outcomes between open and minimally invasive techniques. These findings suggest that the main determinant of surgical approach should be surgeon preference and training.
Introduction
Lumbar spinal fusion is a popular treatment for instability associated with neurological compression and/or spinal deformity [1], and there have been significant advances in the approach, techniques, and implants used. Anterior and lateral methods (anterior lumbar interbody fusion, oblique lumbar interbody fusion, and extreme lateral interbody fusion) are available depending on the problematic level. Several posterior approaches, including posterolateral fusion (PLF), posterolateral lumbar interbody fusion (PLIF), and transforaminal lumbar interbody fusion (TLIF), have also been described. The advent of percutaneous screws led to the concept of minimally invasive approaches designed to reduce blood loss, pain, and length of stay; nevertheless, these techniques may be accompanied by increased intraoperative radiation exposure and a steep learning curve [1,2].
There is considerable debate in the literature regarding the best lumbar spinal fusion approach, and multiple studies and meta-analyses have compared various outcomes for these procedures. However, the heterogeneity of data and recording techniques makes it difficult to draw definitive conclusions. This large single-center retrospective study aimed to assess the perioperative and intermediate-term clinical outcomes of patients with various lumbar fusion techniques at our institution (Salford Royal Hospital, Northern Care Alliance NHS foundation trust). We present perioperative and patient-reported outcome data from consecutive patients who underwent one- or two-level lumbar fusion using either a minimally invasive or open approach with or without interbody cage placement. As a result, this is one of the largest studies comparing different posterior lumbar fusion techniques.
Materials and Methods
1. Patient population
Patient self-reported outcomes were prospectively collected and retrospectively analyzed for patients undergoing one- or two-level lumbar spinal fusion at our institution between January 2011 and December 2018. Demographic data (age, gender, and body mass index [BMI]) for each patient were collected in addition to predominant pre-surgery symptoms (back pain, leg pain/sciatica, and sensory symptoms/motor deficit), surgical indication (e.g., spondylolisthesis, recurrent disc disease, foraminal stenosis, and scoliosis), previous operative history, perioperative outcomes and complications (i.e., post-surgery stay and intraoperative dural tear), and whether later revision surgery was required.
Core outcomes measure index (COMI) questionnaires were used to determine patient self-reported outcome measures. Previous studies have validated the use of COMI questionnaires as a patient-recorded outcome measure, and COMI is the official outcome instrument of the prospectively collected Eurospine Spine Tango database [3,4]. COMI questionnaires were given to patients pre-surgery and at 3 months, 1 year, and 2 years post-surgery. Data were prospectively collected onto the Spine Tango registry by employed clerks independent of the surgical team and were then retrospectively analyzed. The institutional review board at Salford Royal Hospital approved the study, and the requirement for informed consent was waived. The analysis was performed in accordance with the 1964 Helsinki Declaration and its amendments.
2. Surgical procedures
During the study period, our institution used five different lumbar spinal fusion techniques: PLF only, PLIF performed using bilateral muscle strip (PLIF-BMS) or Wiltse approach (PLIF-Wiltse), open TLIF, and minimally invasive TLIF (MI-TLIF).
Cases categorized as PLF underwent pedicle screw instrumentation and fusion using autograft and allograft in the intertransverse plane after decorticating the transverse processes. In PLIF cases, screw instrumentation was also used, and the disc space was accessed by removing bilateral facet joints and inserting bilateral cages packed with autograft and allograft mixture. PLIF cases were subcategorized based on whether the pedicle screws were introduced by a Wiltse approach, followed by smaller midline laminotomy for decompression and cage insertion or traditional bilateral muscle strips from the midline. In most cases, the spinous process, interspinous, and supraspinous ligaments were left intact to retain the posterior tension band. Cases were categorized as TLIF if the access to the disc space was via a single-sided facet joint resection, and fusion was achieved using a single cage through a transforaminal approach. TLIFs were subcategorized based on whether the pedicle screws were inserted via stab incisions (MI-TLIF) or using two paramedian incisions and the Wiltse approach (open TLIF). Facetectomy was performed in MI-TLIF patients via a separate small paramedian incision or by combining the stab incisions for screw insertion on the side from which the cage was inserted.
3. Statistical analysis
Stata ver. 11 (Stata Corp., College Station, TX, USA) and the IBM SPSS statistical software package ver. 25.0 (IBM Corp., Armonk, NY, USA) were used for all statistical analyses. Continuous data were reported as median (±interquartile range) and compared across surgical groups using a Kruskal-Wallis test with Bonferroni correction for multiple comparisons. Pearson’s chi-square test was used to evaluate differences in categorical variables between groups. A repeated measure mixed-effects model with imaging time point as a fixed-effects variable was used to analyze changes in COMI score over time within each surgical procedure group. The Bonferroni method was used for post hoc analysis of pairwise comparisons between different time points.
To evaluate the effect of surgical procedure components on patient-reported outcomes, total COMI scores at each time point were compared across different surgical procedures (PLIF-BMS, PLIF-Wiltse, MI-TLIF, open TLIF, and PLF only) using Kruskal-Wallis test with multiple comparisons. Ordinal logistic regression was used to assess the effect of patient demographics (age, gender, and BMI), surgical indication, operated level, and surgical approach on COMI scores at each pre- and post-surgery time point.
Results
1. Patient demographics
This study included 447 consecutive patients (266 females and 181 males), except for 18 patients undergoing PLIF-BMS (9.13%) and 20 patients undergoing PLIF-Wiltse (16.1%) in which insertion of bilateral cages was not possible due to anatomical limitations and a unilateral interbody cage was used. Demographic features, operative level, preoperative level, and surgical indication are detailed in Table 1. The median age of all patients was 61.7 years (range, 19.8–90.4 years), and patients undergoing MI-TLIF were younger than other surgical approach groups (Kruskal-Wallis test, p=0.03). BMI did not differ significantly between surgical groups (Kruskal-Wallis test, p>0.05). Seventy patients (15.7%) had undergone previous surgery at the index level, which was significantly higher (37.5%) in the PLF-only group (χ2 test, p=0.02) (Table 1). The operated level did not differ significantly (p>0.05) between surgical approaches, and the most common operated levels were L4/5 (58.6% of patients) and L5/S1 (29.5%). Eighteen patients (4%) underwent fusion procedures at more than one adjacent level.

Patient demographics, operated level, and surgical indication stratified by lumbar fusion approach (total=447 patients)
There was no statistically significant difference in the proportion of patients with each symptom (Pearson’s chi-square test, p>0.05). The most common pathological indication for surgery was degenerative spondylolisthesis (54.1% of patients), followed by lytic spondylolisthesis (24.8%) and recurrent disc prolapse (12.3%). There was a significant difference between surgical groups (χ2 test, p=0.03) (Table 1), with 42.0% of MI-TLIF requiring surgery due to a lytic spondylolisthesis compared with 14.3%–29.2% of patients in the other surgical approach groups.
2. Perioperative outcomes
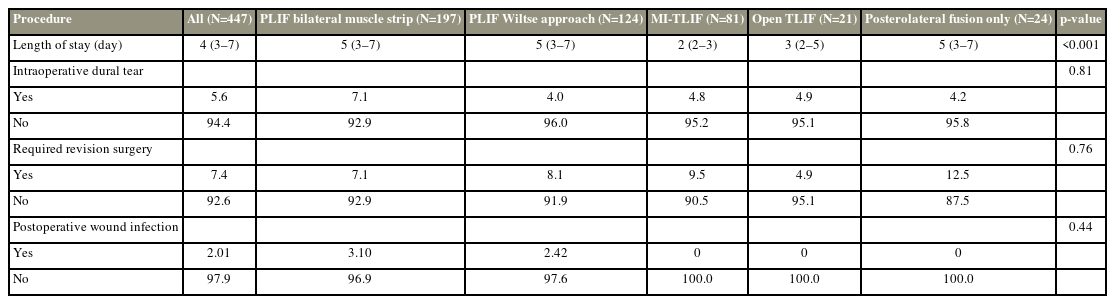
Perioperative measures across different fusion procedures are summarized in Table 2. There was a significantly shorter postoperative stay in the MI-TLIF group (median, 2 days) than in the PLIF-BMS group (Kruskal-Wallis test, p=0.03). There was no significant difference in intraoperative dural tear rate between procedure groups (χ2 test, p>0.05). However, there was a trend to higher rates of dural tear rate in the PLIF-BMS group (7.1%) than in other approaches (4.0%–4.9%). There was no significant difference in intraoperative dural tear rate between different operated levels, first-time procedure patients and patients with previous surgery at the index level, or patients undergoing one- or two-level surgery (χ2 test, p>0.05).
Thirty-three patients (7.4%) required later revision surgery at the operation level. The median time to repeat surgery was 53 days (range, 1–1,946 days). The reasons for repeat surgery were as follows: failure or revision of implanted metalwork (eight patients), repeat decompression at operated level (six patients), extension of fusion construct (five patients), surgical repair of CSF leak/pseudomeningocele (three patients), treatment of post-surgery infection (nine patients), post-surgery hematoma (one patient), and replacement of displaced cage (one patient). There was no statistically significant difference in the repeat surgery rate (χ2 test, p>0.05) (Table 2) or indication for repeat surgery between the different procedure groups. The PLIF groups had severe postoperative wound infections, although the difference between procedures was not statistically significant (χ2 test, p>0.05).
3. Patient self-reported outcomes
All patients included in the study had COMI data recorded for at least one time point. Pre-surgery COMI scores were obtained for 395 patients, 362 patients at 3 months, 326 patients at 1 year, and 354 patients at 2 years. Complete COMI for 116 patients (59.0%) undergoing PLIF-BMS, 65 patients (52.4%) undergoing PLIF-Wiltse, 43 patients (53.1%) undergoing MI-TLIF, 12 patients (57.1%) undergoing open TLIF, and 11 patients (45.8%) undergoing PLF-only were available across all four measurement time points (pre-surgery, 3 months, 1 year, and 2 years post-surgery). COMI scores were lower in all patients (n=447) at each post-surgery time point compared with pretreatment (mixed-effects model, p<0.001) (Fig. 1A). The largest decrease in COMI score was observed in the first 3 months post-surgery (mean difference, −2.59; p<0.001), with a smaller decrease observed between 3 months and 1 year post-surgery (mean difference, −0.84; p<0.001). There was no significant difference in the COMI scores between 1 year and 2 years post-surgery (mean difference, −0.10; p>0.05).
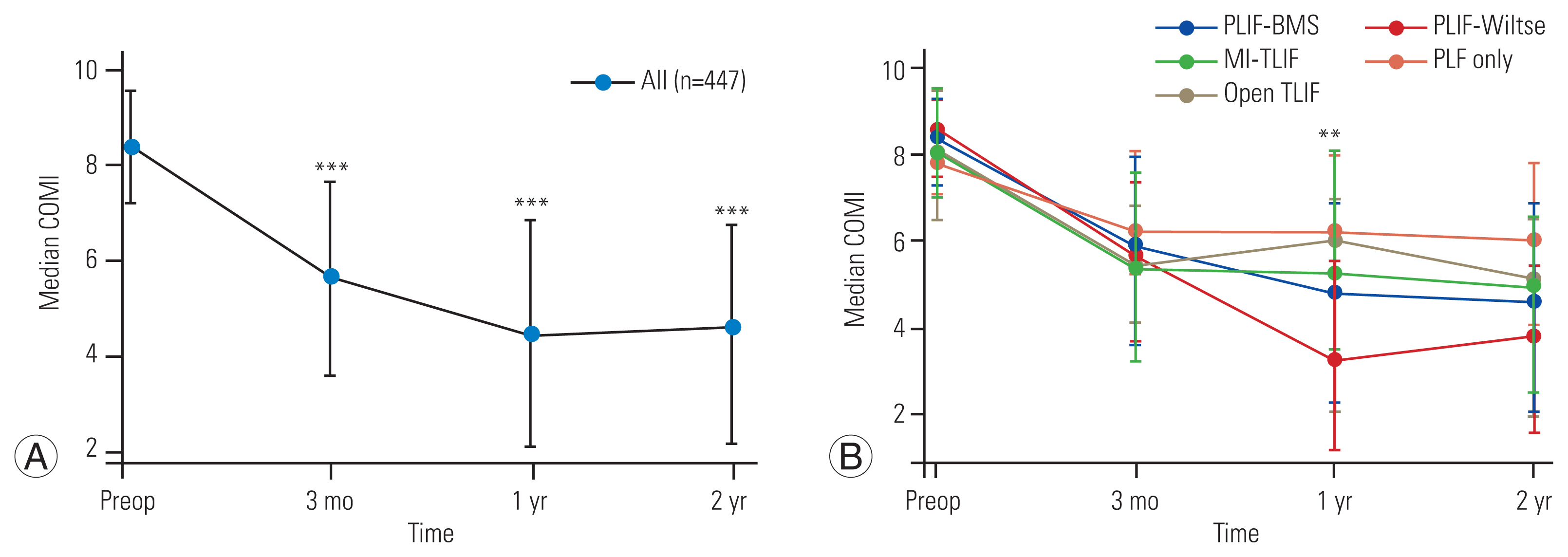
Changes in core outcomes measure index (COMI) score over time stratified by surgical approach. Median and interquartile range of overall COMI score at each timepoint shown. (A) Change in overall COMI score over time for all 447 patients. COMI scores were lower at 3 months, 1 year, and 2 years post-surgery compared to pre-treatment (p<0.001, mixed-effects model). The largest decrease in COMI score was seen in the first 3 months post-surgery. p-value shown represents difference from pre-surgery COMI score and calculated using a repeated measures mixed-effects model with imaging time-point as a fixed-effects variable. Post hoc analysis of pairwise comparisons between different timepoints was performed using the Bonferroni method. (B) Change in overall COMI score stratified by lumbar fusion approach. The p-value shown represents differences in COMI score between surgical approaches at each timepoint. p-value calculated using Kruskal-Wallis test. PLIF-BMS, posterior lumbar interbody fusion with midline posterior approach/bilateral muscle strip; PLIF-Wiltse, PLIF with bilateral Wiltse/muscle splitting approach; MI-TLIF, minimally invasive transforaminal lumbar interbody fusion; Open TLIF, open transforaminal lumbar interbody fusion; PLF-only, pedicle screw/posterolateral fusion only; Preop, preoperative. **p≤0.01. ***p≤0.001.
Separate analysis of the PLIF-BMS, PLIF-Wiltse, and TLIF (minimally invasive and open) surgical groups demonstrated comparable results with decreases in COMI score at 3 months (p≤0.02), 1 year (p≤0.009), and 2 years post-surgery (p≤0.05) compared with pretreatment (mixed-effects model) (Fig. 1B). Significant differences in COMI score were not observed at 3 months in the PLF-only group but were evident at 1 year (p=0.02) and 2 years (mixed-effects model, p<0.001). There was no significant difference in overall COMI scores, either pretreatment or 3 months posttreatment, between the different procedure groups (Table 3). At 1 year, the PLIF-Wiltse group had a significantly lower median COMI score than the MI-TLIF (p=0.02) or PLF-only group (Kruskal-Wallis test, p=0.04) (Fig. 1B). At 2 years post-surgery, there was a trend toward lower overall COMI scores in the PLIF-BMS and PLIF-Wiltse groups than in the TLIF and PLF-only groups, but these differences did not achieve statistical significance (Kruskal-Wallis test, p>0.05).
Subgroup analysis of patients with COMI data at all four time points (n=247) revealed comparable results to the entire cohort, with significant decreases in COMI score (mixed-effects model, p<0.001) at each post-surgery time point (Supplement 1) and no significant difference between procedure groups, except at 1 year post-surgery (Kruskal-Wallis test, p=0.03) (Supplement 2).
4. Subgroup analyses by surgical indication
A subgroup analysis of patients undergoing surgery for degenerative spondylolisthesis exclusively (n=226), the most common surgical indication, was also performed to account for heterogeneity in method and indication for surgery within each surgical approach group. PLIF-BMS and PLIF cases with unilateral rather than bilateral interbody cage implantation were also excluded, and the results of this subgroup analysis are shown in Supplement 3. Significant decreases in COMI score were observed within this degenerative spondylolisthesis subgroup at each posttreatment time point (mixed-effects model, p<0.001) (Supplement 3), and there were no significant differences between procedure groups other than at 1 year post-surgery (Kruskal-Wallis test, p=0.05). Subgroup analyses of patients undergoing surgery for lytic spondylolisthesis and recurrent disc prolapse revealed similar results, with no significant differences between procedure groups at any time point (Kruskal-Wallis test, p>0.05).
Subgroup analyses of patients undergoing surgery for either back pain, leg pain, or lower limb sensory symptoms/motor deficit as the primary preoperative symptom are shown in Supplement 4. Patients who had surgery for leg pain as the primary symptom had a lower overall COMI score at 2 years (Kruskal-Wallis test, p=0.02) than patients who had surgery for back pain or sensory symptoms/motor deficit. COMI scores were lower at each post-surgery time point than pre-surgery within each symptom indication subgroup (mixed-effects model, p<0.001) (Supplement 4), and there were also no significant differences in COMI scores between procedure groups at any postoperative time point (Kruskal-Wallis test, p>0.05).
5. Predictors of preoperative and postoperative patient-reported outcomes
According to univariate ordinal logistic regression, younger age (odds ratio [OR], 0.99; p=0.03) and higher BMI (OR, 1.04; p=0.02) were the only significant independent predictors of higher pre-surgery COMI scores. Younger age was a significant independent predictor of higher COMI scores at 3 months (OR, 0.98; p=0.02) and 2 years (OR, 0.99; p=0.05) postoperatively, and higher BMI was predictive of higher COMI scores at 1 year (OR, 1.06; p=0.003) and 2 years (OR, 1.05; p=0.005) (Table 4). Higher preoperative COMI score was a significant predictor for higher COMI scores at every postoperative time point (p<0.001). Treatment for lytic spondylolisthesis (OR, 1.74; p=0.02) and recurrent disc disease (OR, 2.79; p=0.001) were significant predictors of higher COMI score at 2 years postoperatively (Table 4). The previous index level predicted a higher COMI score at all postoperative time points (p≤0.04) (Table 4). Insertion of bilateral cages was a predictor of lower COMI scores at 1 year (OR, 0.36; p=0.02) and 2 years (OR, 0.43; p=0.03) postoperatively than the use of no cage, in keeping with the observed higher overall COMI scores for the PLF-only group.
Discussion
In this large, single-center study, we investigated the perioperative and intermediate-term clinical outcomes of patients with various posterior lumbar fusion methods. Our findings show that irrespective of the technique used, postoperative COMI scores were lower than pretreatment, with the largest improvement in patient-reported outcomes recorded in the first 3 months post-surgery. Except for patients undergoing PLF without interbody cage placement, who had worse outcomes at 1 year, all lumbar fusion techniques had comparable patient-reported outcomes, and there was no sustained difference in clinical outcomes following open versus minimally invasive techniques at 2 years post-surgery.
Previous studies have demonstrated benefits of minimally invasive TLIF over open T/PLIF with smaller wounds, reduced blood loss, faster recovery, and shorter hospital stay [5–8]. Our center’s findings show that patients receiving MI-TLIF had significantly shorter postoperative visits than open procedure groups. Despite improvements in short-term/perioperative outcomes, our data did not support a significant difference between open and minimally invasive approaches in patient-reported outcome measures, and previous studies and systematic reviews comparing minimally invasive and open lumbar fusion techniques support this finding, demonstrating no differences in intermediate and long-term patient-reported outcome scores [5–7,9].
A key strength of our study is that alongside permitting comparison between minimally invasive TLIF and open P/TLIF, it also allows a comparison between different open fusion procedures with or without using an interbody cage. The main goal of interbody fusion surgery, irrespective of technique, is to decompress neural structures, restore disc height and lumbar lordosis, and stabilize the spine [10]. The comparable patient-reported outcomes across different fusion techniques in this and other studies indicate that, while some techniques, such as those incorporating a muscle-splitting approach and minimally invasive screw insertion, are expected to produce less tissue trauma and shorter postoperative stay [6], this ultimately has little bearing on the intermediate to long-term outcomes of these procedures. Our data showed a nonsignificant tendency toward worse patient-reported outcome measures in patients undergoing solely PLF. Previous research demonstrated that open lumbar fusion procedures integrating interbody cage implantation improve fusion rates, back discomfort, and postoperative functional status in patients with lumbar fusion for spondylolisthesis [11–13]. The fact that we did not analyze rates of radiological fusion or restoration of lumbar lordotic angles is a limitation of our study [14], but likely, the reason for the reduced pain and better functional outcomes in patients undergoing interbody fusion compared with PLF alone is multifactorial, reflecting higher fusion rates, better correction of lumbar lordosis, and more complete foraminal decompression [11–13].
Although there was a trend in our research cohort toward higher rates of revision surgery in the PLF-only group and higher rates of dural tear rate in the PLIF-BMS group, these differences did not reach statistical significance. Although some previous studies have indicated greater revision rates for displaced allografts in MI-TLIF patients [15], other authors have found reduced complication rates when using MI-TLIF [16,17]. The overall revision surgery rate in this analysis was higher than in some recent series [6]. This could be due to differences in the patients and procedures included in the studies, but it could also be due to the extended length of follow-up (>10 years) for some of the earliest recruited patients in our analysis compared with other series. Previous authors have discussed the challenging learning curve associated with minimally invasive fusion, which makes certain complications, particularly those related to instrumentation, more likely [17]. For example, the use of tubular dilator retractors has been proposed to result in an increased incidence of radiculopathy due to poorer visualization of spinal structures and hence less adequate decompression, and this learning curve may explain the discordant findings across the literature in terms of complication rate and revision surgery rate [17].
Although the primary goal of this study was to evaluate the clinical outcomes of patients undergoing different lumbar fusion techniques, our finding that higher BMI was associated with worse preoperative and postoperative patient-reported outcomes is consistent with previous studies [18]. Other authors, however, have shown no clear relationship between BMI and pain outcomes following lumbar surgery, particularly in patients undergoing MI-TLIF [19,20]. Our demonstration that advancing age was not a predictor of poorer postoperative outcomes, with younger patients having worse preoperative and postoperative scores, is consistent with the literature showing equivalent, if not improved, outcomes from lumbar fusion in patients over 65 years [21]. A previous study has also indicated inferior outcomes of lumbar fusion in patients with recurrent degenerative disc disease [22] and lytic spondylolisthesis [23], and our data support this, demonstrating an association with poorer outcome measures at 2 years.
To the best of our knowledge, this is one of the largest single-institution studies comparing patient-reported clinical outcomes across open and minimally invasive lumbar fusion techniques. Other large studies have often reported outcomes at just one postoperative timepoint [6,10], and a key strength of this study is that through a prospective collection of COMI data by a dedicated spinal research team, we were able to achieve assessment of both short-term (3 months) and intermediate-term (1 or 2 years) outcomes. There are, nonetheless, limitations to our study. Recent large series have used propensity-score matching to reduce bias and balance surgical groups on preoperative factors, including age, sex, previous surgery, and preoperative reported outcomes [10]. However, debate in the literature about the use of propensity-score matching for identifying treatment effects [10,24] and the strength of the regression analysis adopted within this study is that alongside interrogating treatment-related effects, it also allowed the identification of other key variables (age, BMI, and preoperative COMI score) that impact on postoperative patient-reported outcomes in addition to interrogating treatment-related effects. An advantage of our single-center study is that collection of patient-reported outcome data, length of stay, and other perioperative outcomes was standardized. However, surgeries were performed across different consultant neurospinal and spinal surgeons. While we were able to compare across different open and minimally invasive lumbar fusion techniques, the effect of individual surgeon technique data was not analyzed. There was also heterogeneity within each surgical approach regarding the technique used and surgical indication. A further source of heterogeneity and potential bias is that complete COMI follow-up data were unavailable for all patients. Within our study, we have sought to account for this heterogeneity through dedicated subgroup analyses of patients with complete COMI data and patients undergoing surgery for the most common surgical indications. These subgroup analyses demonstrate comparable results to the entire study cohort and support our main finding that irrespective of the lumbar fusion approach used, patients improve following surgery with equivalent patient-reported outcomes at 2 years posttreatment.
We do not routinely perform postoperative imaging on lumbar fusion patients with a symptomatic improvement in our center. Therefore, radiographic variables were not evaluated, such as pelvic incidence, pretreatment and posttreatment alignment, restoration of normal lordosis, and correction of focal kyphosis [10]. Therefore, future studies incorporating radiographic data and longer follow-up beyond 2 years to capture later postoperative malalignment should be undertaken.
Conclusions
This is one of the largest single-institution series comparing patient-reported clinical outcomes across open and minimally invasive lumbar fusion techniques and demonstrates that irrespective of the lumbar fusion technique used, patients improve following surgery with generally equivalent outcomes at 2 years posttreatment. These results suggest that surgeon preference and training can reasonably be the main determinant of surgical approach.
Acknowledgments
Thanks go to all the staff at the adult neurosurgery and spinal service at Salford Royal Hospital and to all the staff at the spinal research outcomes team for their help with data collection.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
DL and JL contributed to conception and design of this study. DL, SM, SS, and JL contributed to the data acquisition. DL, SM, RC, SS, and JL contributed to the data analysis, interpretation, and statistical analysis. DL, SM, RC, SS, and JL contributed to the writing and review of this manuscript.
Supplementary Materials
Supplementary materials can be available from https://doi.org/10.31616/2022.0448.
Supplement 1.
Changes in patients self-reported outcomes score in subgroup of patients with complete COMI data at all four timepoints (n=247).
Supplement 2.
Patient self-reported outcomes stratified by lumbar fusion approach.
Supplement 3.
Changes in patients self-reported outcomes score in subgroup of patients undergoing surgery for degenerative spondylolisthesis only (n=226).
Supplement 4.
Changes in patients self-reported outcomes stratified by primary (predominant).