Current Trends in Artificial Intelligence-Assisted Spine Surgery: A Systematic Review
Article information
Abstract
This systematic review summarizes existing evidence and outlines the benefits of artificial intelligence-assisted spine surgery. The popularity of artificial intelligence has grown significantly, demonstrating its benefits in computer-assisted surgery and advancements in spinal treatment. This study adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses), a set of reporting guidelines specifically designed for systematic reviews and meta-analyses. The search strategy used Medical Subject Headings (MeSH) terms, including “MeSH (Artificial intelligence),” “Spine” AND “Spinal” filters, in the last 10 years, and English—from January 1, 2013, to October 31, 2023. In total, 442 articles fulfilled the first screening criteria. A detailed analysis of those articles identified 220 that matched the criteria, of which 11 were considered appropriate for this analysis after applying the complete inclusion and exclusion criteria. In total, 11 studies met the eligibility criteria. Analysis of these studies revealed the types of artificial intelligence-assisted spine surgery. No evidence suggests the superiority of assisted spine surgery with or without artificial intelligence in terms of outcomes. In terms of feasibility, accuracy, safety, and facilitating lower patient radiation exposure compared with standard fluoroscopic guidance, artificial intelligence-assisted spine surgery produced satisfactory and superior outcomes. The incorporation of artificial intelligence with augmented and virtual reality appears promising, with the potential to enhance surgeon proficiency and overall surgical safety.
Introduction
Artificial intelligence (AI) has significantly influenced the healthcare sector [1,2]. The contemporary definition of AI emphasizes the application of algorithms, enabling machines to address problems that traditionally necessitate human intelligence [3]. In general, AI is designed to recognize patterns through algorithms capable of self-correction and continuous improvement. AI is proficient in performing human cognitive functions, including image, word, and pattern recognition, and decision making with integrated learning and improvement [4]. Moreover, AI algorithms excel in identifying correlations among numerous variables, thereby establishing relationships that might escape human comprehension [1].
Currently, the use of AI in spine surgery is in its early developmental phase, encountering several obstacles such as fragmented data, insufficient integration, and limited clinical implementation [5]. However, as AI is being applied across the entire medical field, AI-based research in challenging and high-risk spinal surgeries is ongoing. The relative complexity and higher surgical risks associated with spinal surgeries make them a focal point for ongoing studies in this area [6].
This systematic review offers a comprehensive overview of the most clinically relevant applications of and current trends in AI in spine surgery. Accordingly, this study aimed to focus more on how AI can be applied in the surgical process. Before the review, a brief overview of the types of AI commonly used in spine surgery is presented for a comprehensive understanding of the content. This systematic review summarizes existing evidence and outlines the benefits and types of AI in spine surgery.
Methods
1. Literature search strategy
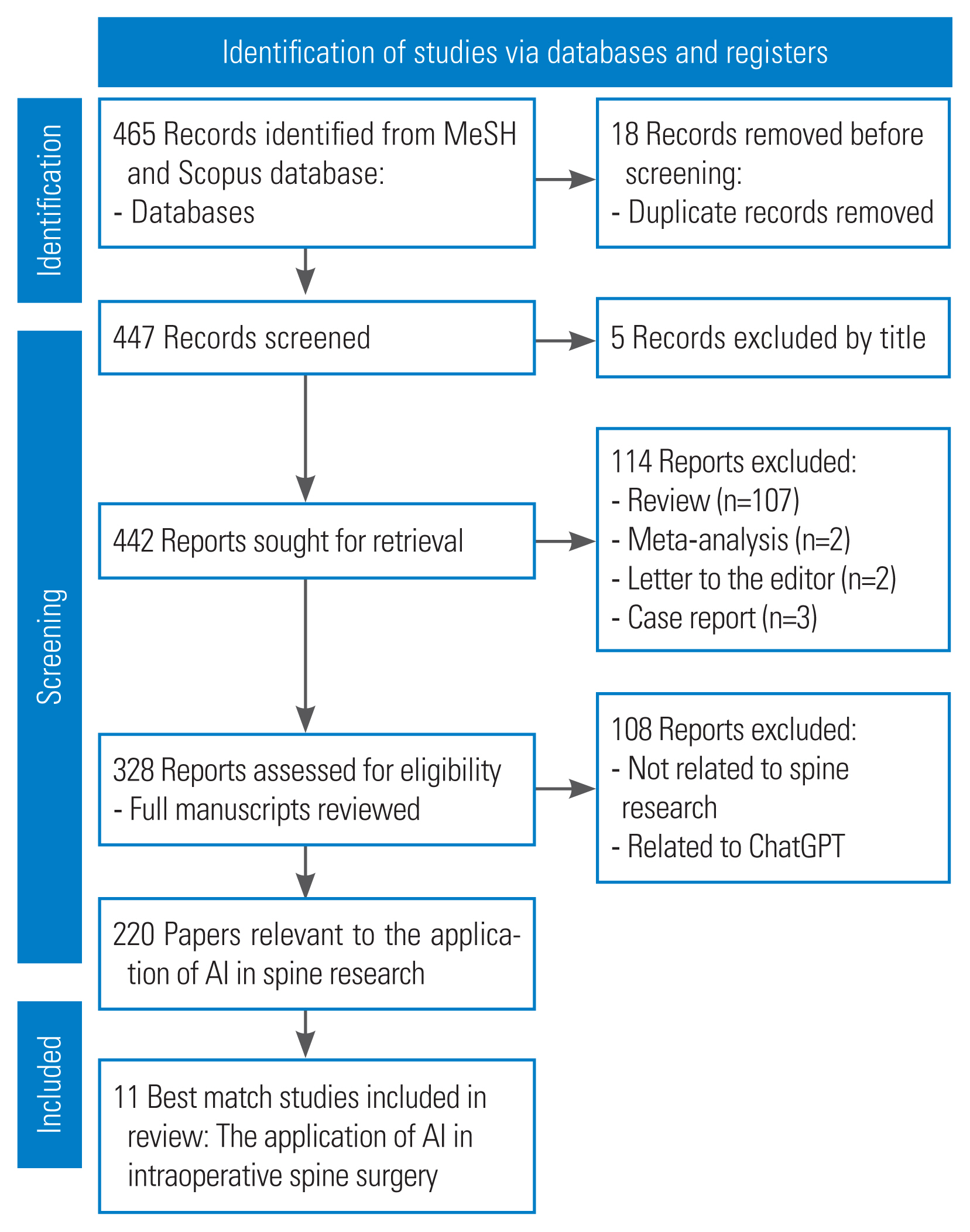
An extensive literature search was performed on the PubMed and Scopus databases to examine existing research on the application of AI in spine surgery. This systematic review was conducted following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) criteria (Fig. 1). A literature search was performed using the search engine to collect articles published in PubMed and Scopus between January 1, 2013, and October 31, 2023, using MeSH (Medical Subject Headings) terms. The search included the keywords “AI,” “spine,” and “spinal” in the title or abstract. Additional manual checks of the reference lists were also performed. Only articles written in English were considered for inclusion.
2. Inclusion and exclusion criteria
This systematic review included all randomized controlled trials (RCTs) and observational cohort studies. No limitations were set on the research design, such as retrospective or prospective. Research from other domains, case reports, reviews, meta-analyses, and studies lacking available abstracts or full texts were excluded. Two independent reviewers conducted the literature collection, resolving any discrepancies through consensus.
3. Data extraction
The following data were recorded: (1) author and year of publication, (2) study design, (3) country in which AI was conducted, (4) type of AI or algorithm technique, (5) AI method, (6) main objectives, (7) objective of findings, and (8) other details of the study.
4. Assessment of the risk of bias in this systematic review
The risk of bias for each RCT was assessed, including selection, performance, attrition, detection, and reporting biases. Each prejudice was categorized as either high, low, or unknown risk. Before comparing their findings, two investigators independently assessed the degree of bias in the included studies. If disagreements existed, judgments were made based on consensus, and if needed, the opinion of a third author was sought. Furthermore, a third reviewer successfully addressed any lingering discrepancies in the assessment of the obtained data.
Results
A total of 442 articles that met the first screening criteria were identified in PubMed and Scopus. An extensive examination revealed 220 papers that met the specified criteria, of which 11 were deemed suitable for inclusion in the analysis after comprehensive inclusion and exclusion criteria. This study focused on the purpose of AI and the type of study, considering the nationality of the participants. The research conducted by the author in the published year analyzed demographic data (Table 1).
1. Study design and publication information
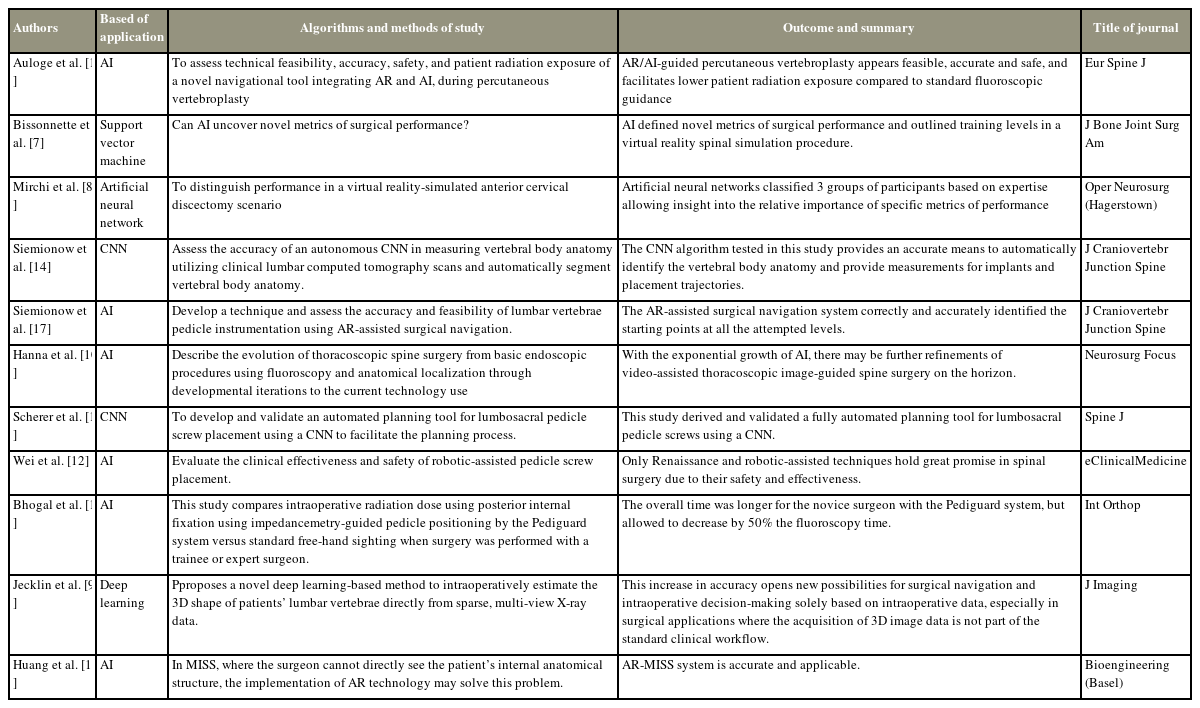
In this review, prospective and retrospective studies were identified. Three studies were prospective studies by Bissonnette et al. [7], Mirchi et al. [8], and Jecklin et al. [9]. Two were retrospective studies by Hanna et al. [10] and Scherer et al. [11]. Two were experimental studies by Wei et al. [12] and Huang et al. [13]. Two cadaveric studies were identified by Siemionow et al. [14]. Only the RCTs by Auloge et al. [15] and Bhogal et al. [16] were identified.
2. Nationality
The number of publications on AI-assisted surgery increased between January 1, 2013, and October 31, 2023, as the trend toward this AI technique grew. These studies included three articles from the United States, two each from Canada and China, and one each from France, Germany, Belgium, and Switzerland.
3. Risk of bias analysis
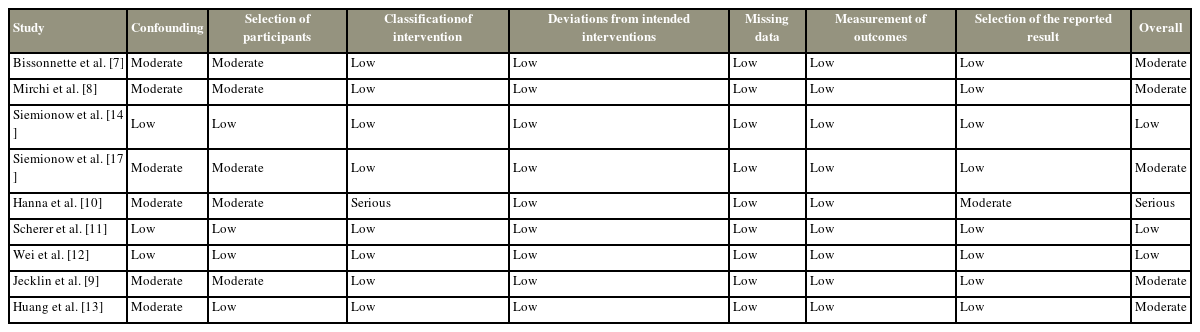
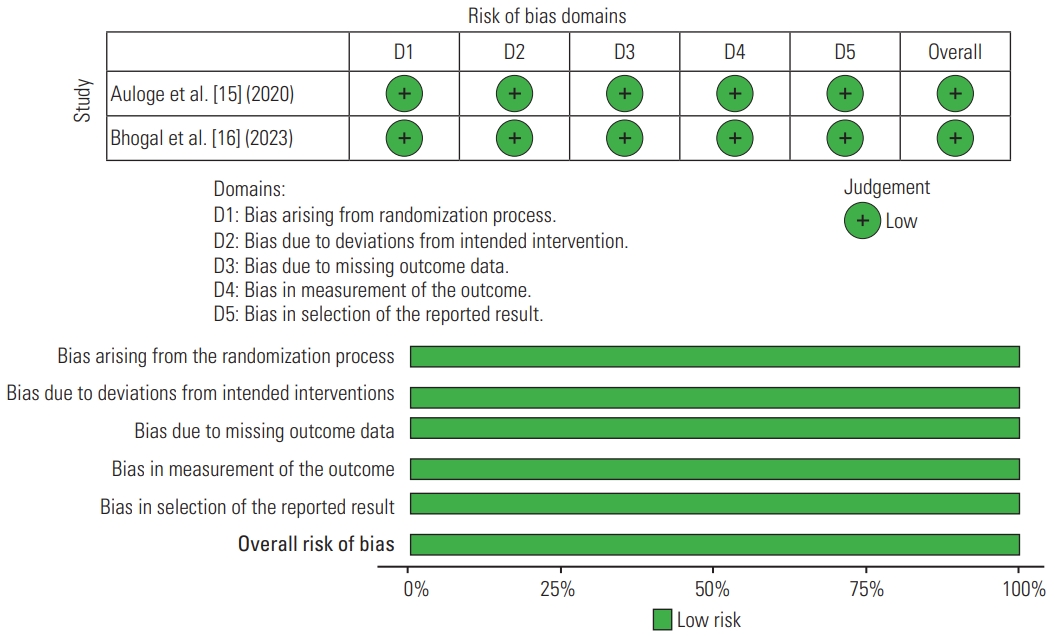
In this systematic review, a summary of the risk for non-RCT bias is shown in Table 2, and RCT bias is shown in Table 3. The risk of bias in non-RCT studies was assessed using the ROBINS-I tool (Cochrane, London, UK) [17] (Fig. 2). For RCT studies, the risk of bias was evaluated using the Cochrane Risk of Bias Assessment Tool (Cochrane) [18] (Fig. 3). For the non-RCT studies, three had a low risk of bias, five had a moderate risk, and only one study had a serious risk (Table 2). All RCT studies had a low risk of performance bias for blinding of outcome assessment, incomplete outcome data, and selection outcome reporting (Table 3).

Risk of bias summary for non-randomized controlled trials, assessed using the risk of bias in non-randomized studies of interventions ROBINS-I tool (Cochrane, London, UK).
4. Types of AI commonly used in spine surgery
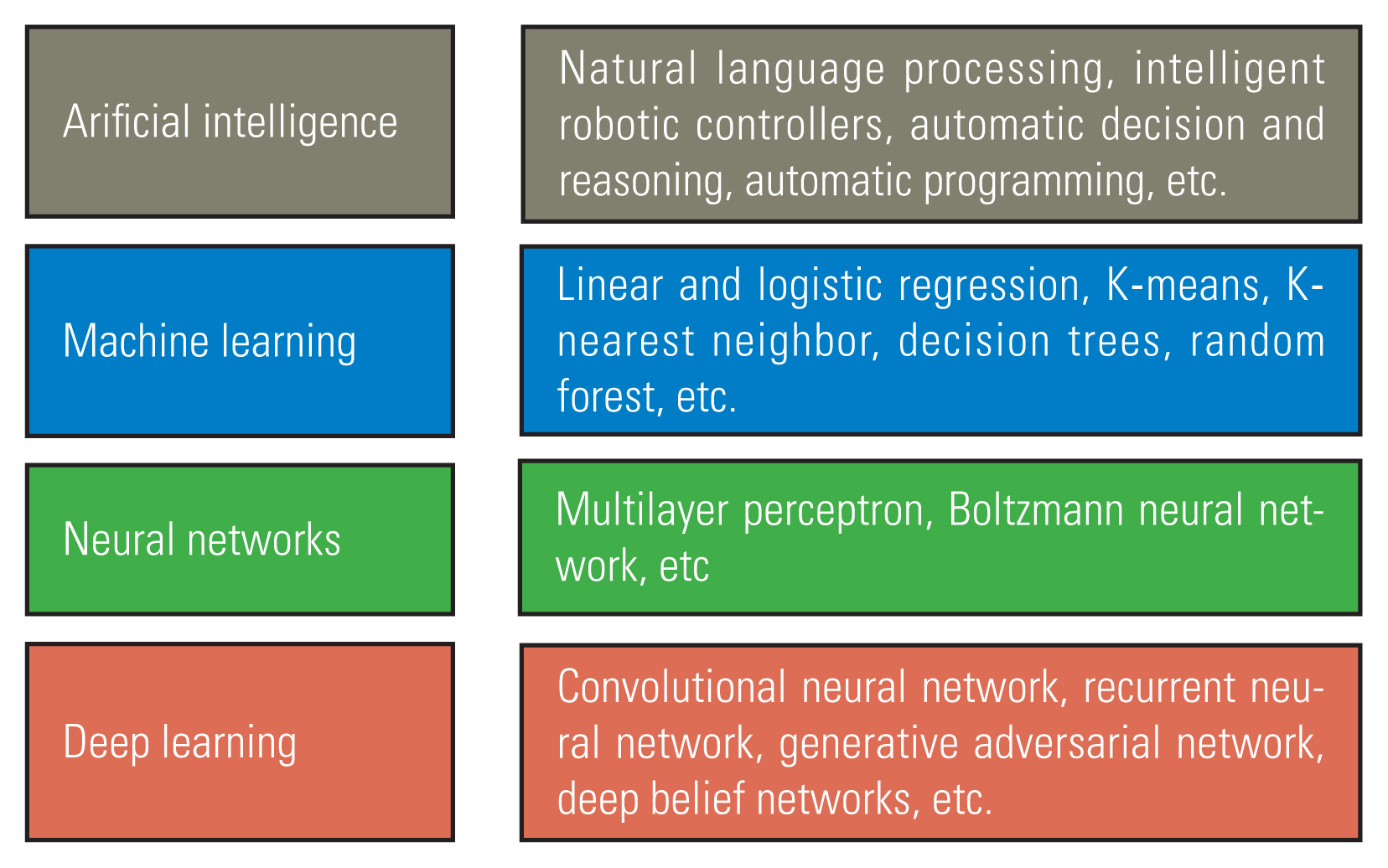
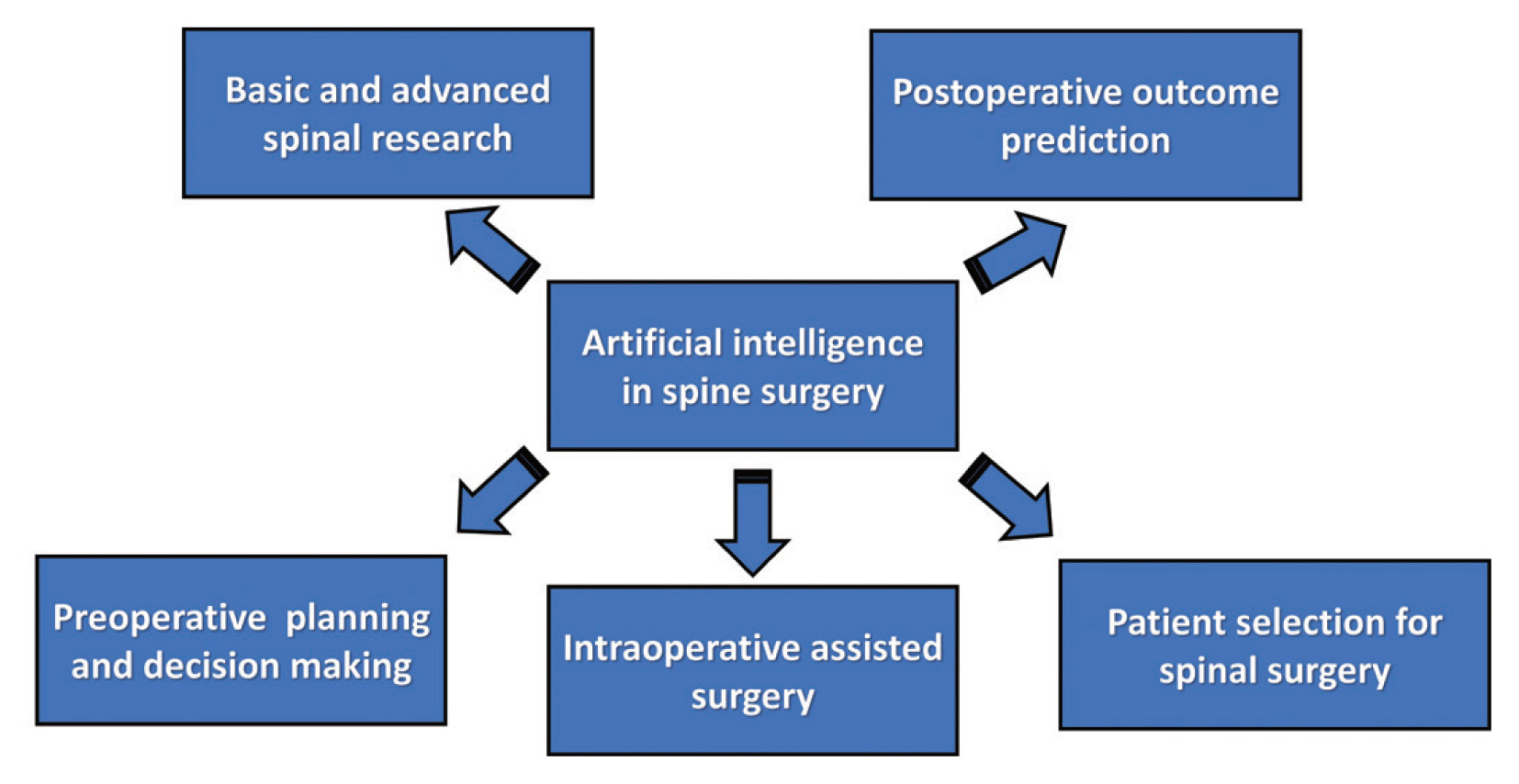
AI is a broad generic term. It encompasses various forms of automated decision making [19]. Embedded within the realm of AI is a hierarchical structure of processes, each exhibiting escalating levels of sophistication. Various AI types fall into either narrow or general categories, depending on their capacity to replicate human functions [1]. AI, machine learning (ML), and deep learning (DL) have emerged as highly discussed topics in the field of spine surgery. AI refers to the development of intelligent machines with advanced cognitive abilities. ML is a branch of AI that enables the development of applications powered by AI. DL is a type of ML that employs large amounts of data and intricate algorithms to train a model [1,20]. Fundamental differences, relationships, and examples of AI are shown in Fig. 4. AI in spine surgery includes basic spinal research, preoperative planning, decision making, postoperative outcome predictions, intraoperative navigation-assisted surgery, and selection of patients for spinal surgery (Fig. 5) [5,21–26].
1) ML
ML, a subset of AI, involves training models to make predictions based on known datasets, allowing the machine to “learn” from the data [21,27]. Supervised ML models use labeled input data to establish relationships with output training data, which are commonly employed for data classification or prediction tasks [21,28,29]. In contrast, unsupervised ML models operate on unlabeled raw training data, identifying relationships and patterns within the dataset and uncovering inherent trends [29]. Unsupervised models serve primarily as efficient representations of the initial dataset, using statistical properties such as densities, distances, or clustering to enhance the comprehension of relationships or patterns within the data [21,30]. Supervised ML models are shown in Fig. 6.
2) DL
DL represents a higher level of abstraction than unsupervised ML because it operates without labeling the input or output variables [1]. This approach involves emulating the neural connections found in the human brain. DL encompasses a collection of ML methods and leverages techniques such as artificial neural networks (ANNs) and convolutional neural networks (CNNs) [6,22,25,26].
3) NLP
Natural language processing (NLP) is a dedicated discipline within AI, focusing on the comprehension of human language [31]. NLP plays a pivotal role in the extensive, plain-text analysis of electronic medical records. NLP takes unstructured data and goes beyond mere word recognition, incorporating syntax and semantics to extract meaning and sentiment [1]. NLP has utility in diverse applications, including the generation of medical records from speech, summarizing historical clinical notes, handling billing processes, scheduling surgeries, and facilitating triage [1,32–34].
4) Computer vision
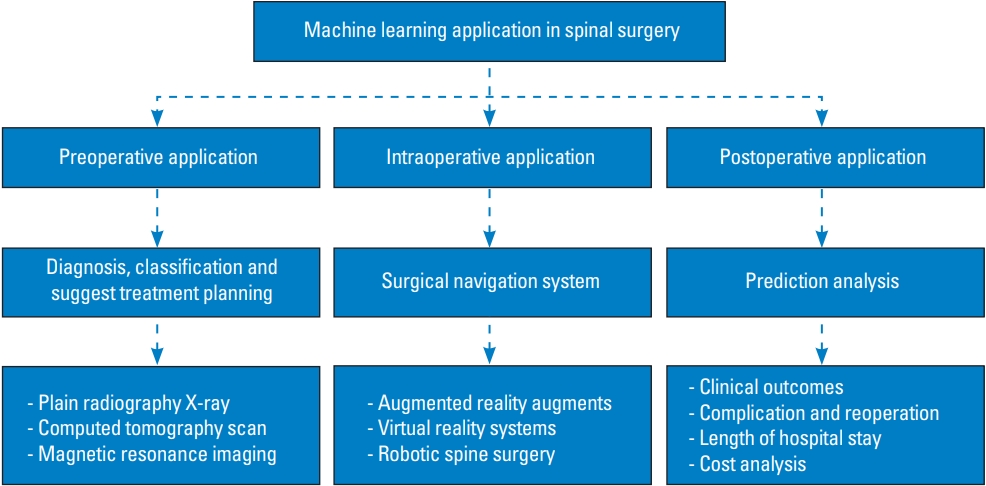
Computer vision is the most well-known application of DL and commonly uses CNNs for pattern recognition [1,35]. CNNs are predominantly used in healthcare for image-based diagnosis. CNNs consist of nodes, each assigned specific weights. However, the connections between layers are more constrained. This convolution process enables computers to interpret images in a manner analogous to that of the human brain. We summarized computer vision for assisted navigation surgery for intraoperative application (Fig. 7).
Discussion
AI applied in spinal surgery is evolving toward ultimate integration with augmented reality (AR) and virtual reality (VR). This development involves the direct application of AI in surgery, starting from preoperative learning and evaluating the surgeon’s capabilities to assist in surgery under navigation for enhanced safety, precision, and speed. In recent years, various AI instruments, including surgical robots, navigation systems, and computer-assisted surgical (CAS) systems, have experienced rapid development in orthopedic surgery [36].
VR surgical simulators create a secure setting for trainees to engage in targeted surgical scenarios, fostering self-guided learning [37]. The use of AI technology, including ANNs, holds promise in processing extensive datasets from simulators. This enables a deeper understanding of the significance of specific performance metrics during simulated operative tasks. Bissonnette et al. [7] investigated the use of ML to evaluate surgical expertise in a VR spine procedure, specifically focusing on VR hemilaminectomy. They processed raw data from the VR hemilaminectomy process through metric extraction, normalization, and selection before conducting ML analysis. Upon identifying the optimal algorithm and parameters, the researchers trained a unified model using all available data and subjected it to generalizability testing on new subjects. Using this approach, they assessed the safety, efficiency, tool motion, and coordination of the procedure. They concluded that AI could play a role in the assessment of surgical competence. In addition, a study evaluated the performance of anterior cervical discectomy and fusion (ACDF) using the concept of ANNs [8]. ACDF has historically been widely used and has proven to be safe and effective [38]. Considering the complications that may arise from surgery, it is undoubtedly a challenging procedure for novice surgeons. However, understanding the areas where one lacks proficiency in the surgical process before surgery can be of great assistance in preparing for surgery. Mirchi et al. [8] analyzed 369 metrics in four areas (safety, efficiency, motion, and cognition) obtained from 21 participants who underwent anterior cervical discectomy using VR. They employed an ANN for the analysis. Among these metrics, those related to safety were identified as the most crucial aspect in areas demonstrating surgical competence. The importance of both these studies lies in the potential synergy of integrating VR simulation and AI. This combination offers the prospect of safer training and objective assessment of surgical skills, ultimately contributing to enhanced patient care.
Spinal surgeries pose significant challenges because of their technical intricacy and proximity to critical organs such as the spinal cord, nerves, and aorta [9,39,40]. Previous studies have identified various surgeon- and patient-specific factors as potential causes of postoperative complications in these interventions, ranging from pedicle screw malplacement to cage malpositioning [41–44]. To address these challenges, CAS navigation techniques have been introduced [9]. These techniques facilitate and standardize spinal procedures by offering three-dimensional (3D) intraoperative spatial guidance. Studies have demonstrated that CAS navigation leads to substantially higher accuracy in pedicle screw insertion than free-hand and conventional two-dimensional fluoroscopy-guided methods [42,45,46]. Moreover, CAS technologies have been employed for cage insertion, resulting in improved performance [47,48]. This integration of CAS navigation holds promise for enhancing the precision and safety of spinal interventions [49]. In particular, in the current prevalence of minimally invasive spine surgery (MISS), the use of navigation provides an advantage by enhancing the visibility of the intricate anatomical structures of the challenging spine. This makes surgery more comfortable, given the complexities involved in MISS. In critical surgeries where the trajectory, such as pedicle screw insertion or vertebroplasty, is significant, certain studies have used AI to automatically predict the trajectory [11,12,15].
The most prevalent CAS systems still depend on preoperative 3D imaging and preoperative planning [9,50]. This process enables the creation of patient-specific models, often referred to as biomechanical digital twins, which serve as the foundation for computer simulations [9]. These simulations aid in determining and optimizing surgical plans. However, the registration of preoperative data to the actual anatomy remains a challenging and occasionally error-prone task, often requiring meticulous manual input [51,52]. The need for a registration process between preoperative and intraoperative data remains a significant bottleneck in the widespread adoption of CAS systems [9]. To overcome these drawbacks, recent research has explored the transformation of 3D images of the spine based on X-ray images captured during surgery, rather than relying solely on preoperative images [9]. Jecklin et al. [9] introduced an innovative DL approach designed to estimate the 3D shape of patients’ lumbar vertebrae in real-time using sparse, multiview X-ray data during surgery. This study marks a significant advancement, offering new prospects for surgical navigation and intraoperative decision making exclusively using real-time data. This is particularly valuable in surgical scenarios where obtaining 3D image data is not a standard part of the clinical workflow.
In MISS, where the surgeon cannot directly observe the patient’s internal anatomical structure, the implementation of AR technology may solve this problem [13,53]. Currently, in navigation spine surgery, there is a shift from performing surgery on two-dimensional monitors toward the adoption of AR. Many studies in this area have highlighted the central role of AI in data analysis and prediction, emphasizing its significance in advancing the field [10,13,14,54]. Huang et al. [13] integrated AR, AI, and optical tracking to improve the AR-MISS system. The system encompasses three key functions: AR radiograph superimposition, AR real-time puncture needle tracking, and AR intraoperative navigation. Through AR technology, the system enables the convergence of three spaces: the actual intraoperative, video image, and medical image spaces. This integration allows surgeons to directly access information from all three spaces simultaneously, eliminating the need to divert their gaze from the surgical site. Siemionow et al. [54] pioneered a technique that evaluated the precision and viability of lumbar vertebrae pedicle instrumentation with AR-assisted surgical navigation (ARAI). The ARAI surgical navigation system successfully and accurately identified the starting points at all attempted levels. Furthermore, the VR image overlay precisely matched the actual anatomy in all tested scenarios.
Recently, not only through the integration of AI into navigation but also by leveraging the proprietary characteristics of bone conductivity called “Pediguard,” a tool has been developed to assess the penetration of pedicle screws without relying on fluoroscopy [16]. This device functions as a wireless perforation instrument, similar to a conventional pedicle awl, and operates based on the principle of local tissue electrical conductivity, which is measured through the electromagnetic field at the instrument’s tip. The sharp tip of the instrument features an electronic conductivity sensor that translates relative electronic conductivity values into an audible signal. Through ML, electronic conductivity analysis allows the assessment of whether the pedicle has been penetrated. Changes in electrical conductivity at the distal part of the instrument alert the surgeon through a change in sound. This detection is possible because alterations in the electromagnetic field around the instrument tip enable the identification of media with a consistency different from that of the bone (Table 4). This systematic review provides a thorough and inclusive summary of the entire field of research. The authors propose and approve the current advances in AI in spine surgery (Table 5).
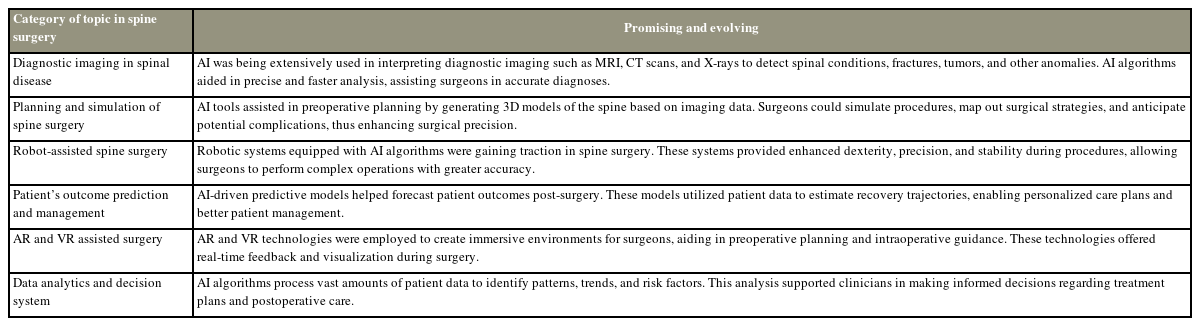
Summary of the advancements in artificial intelligence (AI) in the field of spine surgery, which showed great potential and were continuously developing
The importance of AI in healthcare is expected to increase, contributing to personalized medicine, illness diagnosis, medication development, and therapy optimization. The use of AI-driven technology is expected to significantly transform patient care via the provision of more accurate diagnoses and personalized treatment regimens. However, progress in AI is expected to result in the creation of increasingly advanced autonomous systems and robots. These technologies can be used in diverse sectors such as manufacturing, transportation, agriculture, and healthcare, enabling the execution of intricate tasks with less human involvement.
Conclusions
In complex spinal surgeries characterized by challenging anatomical structures and relatively high surgical difficulty, AI is used in various ways throughout perioperative procedures. Particularly during the intraoperative phase, its integration with AR and VR appears promising, as it can enhance surgeons’ skills and contribute to increased surgical safety.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection, and analysis were performed by WL, PS, and STC. The first draft of the article was written by WL and STC. All authors commented on previous versions of the article. All authors read and approved the final article.