Effect of Furosemide on Prevertebral Soft Tissue Swelling after Anterior Cervical Fusion: A Comparative Study with Dexamethasone
Article information
Abstract
Study Design
Retrospective cohort study.
Purpose
This study aimed to investigate the effect of furosemide on prevertebral soft tissue swelling (PSTS) after anterior cervical fusion (ACF) and compare it with the effect of dexamethasone.
Overview of Literature
Postoperative PSTS is a common complication of ACF. Dexamethasone has been used for its treatment; however, its efficacy remains controversial. Furosemide may reduce PSTS if it is soft tissue edema; however, no studies have demonstrated the effect of furosemide on PSTS after ACF.
Methods
The symptomatic PSTS group received intravenous (IV) administration of dexamethasone or furosemide. The asymptomatic PSTS group did not receive any medication. Patients were divided into the control (no medication, n=31), Dexa (IV dexamethasone, n=25), and Furo (IV furosemide, n=28) groups. PSTS was checked daily with simple radiographs and medication-induced reductions in PSTS from its peak or after medication.
Results
The peak time (postoperative days) of PSTS in the control (2.27±0.47, p<0.05) and Dexa (1.91±0.54, p<0.01) groups were significantly later than that in the Furo group (1.38±0.74). PSTS was significantly lower in the Furo group than in the Dexa group from postoperative days 4 to 7 (p<0.05). PSTS reduction after the peak was significantly greater in the Furo group than in the control (p<0.01) and Dexa (p<0.01) groups. After starting the medication therapy, the Furo group showed a significantly greater reduction in PSTS than the Dexa group (p<0.01). No difference was found in symptom improvement among the three groups.
Conclusions
If furosemide is used to reduce PSTS after ACF, it can effectively reduce symptoms.
Introduction
Anterior cervical fusion (ACF) is one of the most widely performed surgical procedures for the treatment of degenerative cervical spine diseases by directly decompressing the offending lesion [1]. Although ACF is a relatively safe technique, prevertebral soft tissue swelling (PSTS) can frequently cause discomfort to patients after ACF [2,3]. When the pharynx and esophagus are retracted laterally, local perfusion in their walls is diminished, leading to postprocedural hyperemia, which may be the underlying mechanism of postoperative PSTS [4].
PSTS complications vary from mild, such as transient sore throat and dysphagia, to severe, such as esophageal perforation and airway obstruction [2]. Swallowing difficulty was reported to occur in 60% of patients and persisted for >6 months in 12% [5]. Furthermore, airway complications were reported in 1.7%–6.0% of patients [2,5,6] and 1.7%–2.8% of patients with airway problems that required reintubation because of severe airway obstruction [6].
Previous studies have reported that PSTS peaked 2–3 days postoperatively and lasted for at least 2 weeks [7,8]. Therefore, some studies have recommended continuing intubation or delaying extubation after surgery to reduce PSTS complications [6,7]. PSTS was more significant at the C2, C3, and C4 levels than at the C5, C6, and C7 levels [7].
Despite studies on PSTS prevention or treatment, no treatment methods have been standardized so far [9]. Intravenous steroid injection is the most common therapeutic intervention for PSTS; however, its efficacy remains controversial [10].
Furosemide, a loop diuretic, is commonly used to treat edema associated with cardiac, renal, and hepatic failure. Since PSTS is predominantly an edematous condition of soft tissues [4], it may be managed effectively with furosemide. However, no study has examined the effect of furosemide on PSTS after ACF. In this study, we aimed to investigate the effect of furosemide on PSTS and compare it with the therapeutic effect of dexamethasone.
Materials and Methods
The study was approved by the ethics committee of Chung-Ang University Hospital (approval no., 2104-014-19366). Of the 126 patients who underwent ACF between January 2017 and June 2020, 94 were subjected to daily diagnostic radiography. The study included patients who underwent ACF at one or two levels because of degenerative diseases such as disk herniation, ossification of the longitudinal ligament, or cervical stenosis with symptoms of cervical myelopathy or radiculopathy.
Of the 94 patients, six who had fractures, three who underwent corpectomy, and one who had a history of a thyroid operation were excluded. The remaining 84 patients were enrolled in this study.
In each case, a right-sided Smith-Robinson anterior cervical approach was used. Complete discectomy, osteophyte removal, and endplate preparation were performed; however, uncovertebrectomy was not performed. In all patients, Zero-P anchored cages (DePuy Synthes, Zuchwil, Switzerland) were used, and the cages were filled with demineralized bone matrix as a bone graft substitute. The surgical procedures were performed by three spinal surgeons.
Patients were divided into the control (n=31, no medication), Dexa (n=25, treated with dexamethasone), and Furo (n=28, treated with furosemide) groups.
The medications were started when the patients showed PSTS signs combined with symptoms, such as swallowing difficulty or dyspnea. The decision regarding the commencement of medication and its timing was at the sole discretion of the attending physician. If patients with mild symptoms were expected to recover well without additional treatment, dexamethasone or furosemide was not prescribed. The Dexa group received 5 mg of dexamethasone intravenously every 8 hours. The Furo group received 20 mg of furosemide intravenously twice a day [11]. Dexamethasone was used from January 2017 to December 2018, and furosemide was subsequently started according to our modified treatment protocol from January 2019 to June 2020. The medications were discontinued when relevant symptoms were relieved.
1. Radiographic measurement
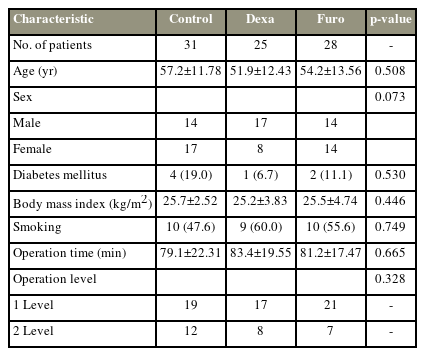
To measure PSTS, a parallel line was drawn from the upper endplate of the lower vertebral body at the surgical level, and the thickness of the soft tissue in front of the one-level ACF was measured (Fig. 1). In two-level ACF, the measurement was made at the upper endplate of the vertebral body between the two operated discs. These measurements were obtained on simple lateral radiographs taken preoperatively and daily until day 7 after surgery (Fig. 2).
Measurement of the prevertebral soft tissue. A line parallel to the upper endplate of the vertebral body was drawn and the diameter of the soft tissue was measured. (A) In the case of one-level anterior cervical fusion (ACF), the measurement was made at the upper endplate of the lower vertebral body of the operated disc. (B) In the case of two-level ACF, the measurement was made at the upper endplate of the vertebral body between the two operated discs.
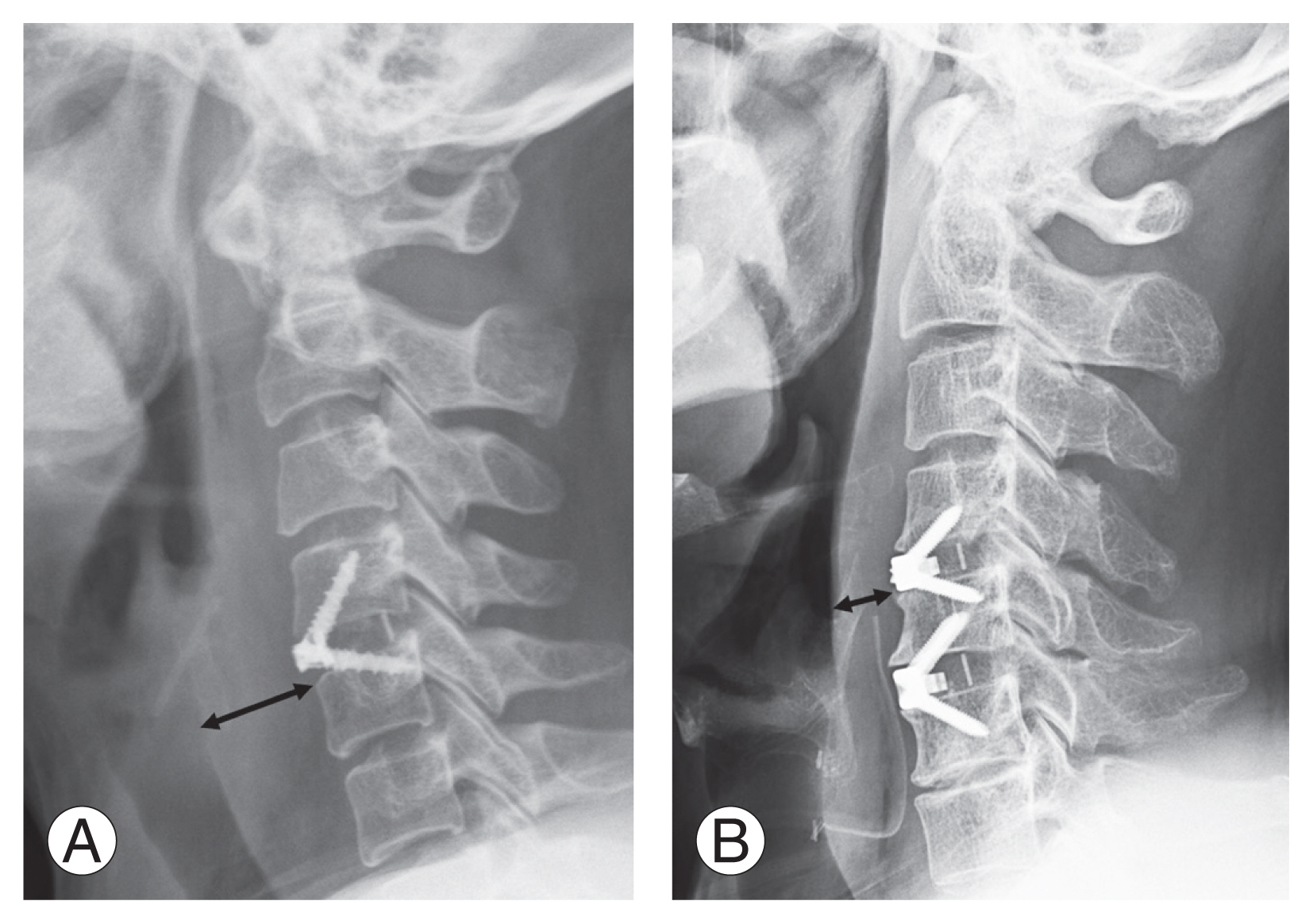
Daily changes of prevertebral soft tissue swelling (PSTS) at the center of the surgery level. Values are presented as mean±standard deviation. Dexa, dexamethasone; Furo, furosemide; Preop, preoperative; POD, postoperative day. *p<0.05. **p<0.01.
PSTS reduction was defined as the difference between the peak PSTS and subsequent PSTS. The reductions in PSTS on days 1, 2, 3, 4, and 5 after the peak PSTS day (peak+1, peak+2, peak+3, peak+4, and peak+5, respectively) were measured (Fig. 3). The medication effects were checked by the amount of PSTS reduction in the Dexa and Furo groups on days 1, 2, 3, 4, and 5 after starting the medications (Med+1, Med+2, Med+3, Med+4, and Med+5, respectively) (Fig. 4).

Reduction in the prevertebral soft tissue swelling (PSTS) from the peak point. Values are presented as mean±standard deviation. Dexa, dexamethasone; Furo, furosemide; Peak+n, n days after surgery. **p<0.01.
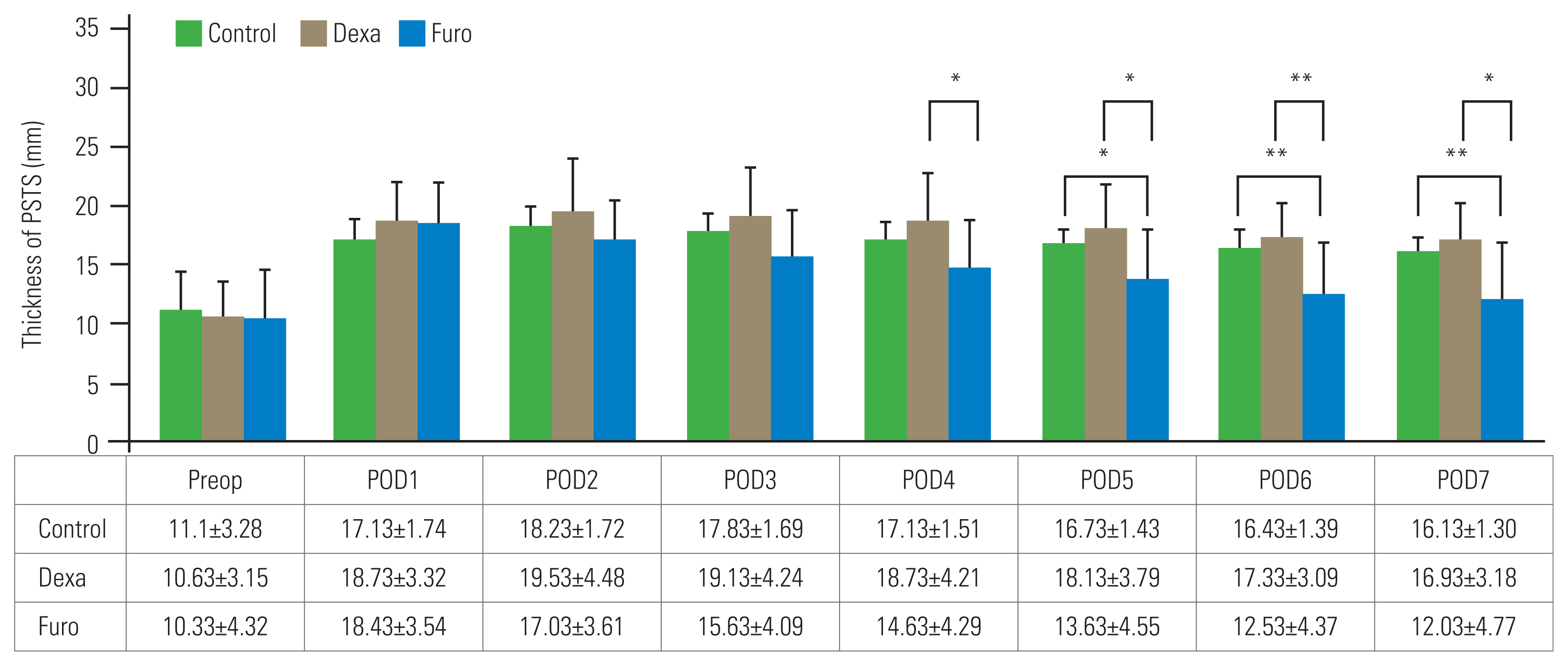
Reduction in the prevertebral soft tissue swelling (PSTS) after medication. Values are presented as mean±standard deviation. Dexa, dexamethasone; Furo, furosemide; Med+n, n days after medication. **p<0.01. ***p<0.001.
Radiological measurements were performed using the picture-archiving communication system of our hospital. Two neurosurgeons (J.S.J. and M.J.K.) made two independent observations at an interval of at least 2 weeks, and the mean value was used in the study. The intra- and inter-observer intraclass correlation coefficients (ICCs) were examined to determine the reliability of the radiological measurements of PSTS.
2. Clinical analysis
The Visual Analog Scale (VAS) was used to measure the severity of odynophagia. A single assessment of the symptoms was conducted on postoperative day (POD) 7.
3. Statistical analysis
The radiological measurements of PSTS were analyzed using Student t-test for comparisons between the two groups. The sex ratio, number of patients with diabetes mellitus, smokers, and number of operation levels were analyzed using the chi-square test. The age and body mass index were analyzed with Student t-test. A p-value <0.05 denoted statistical significance. The ICCs for radiological measurements were graded using previously described semiquantitative criteria (i.e., 0.90–1.0, excellent; 0.70–0.89, good; 0.50–0.69, fair/moderate; 0.25–0.49, low; and 0.0–0.24, poor) [12].
Results
No significant differences were found in the demographic data among the three groups (Table 1). Moreover, no significant difference was noted on the day of starting the medications (1.3±0.49 days and 1.3±0.57 days, respectively, after surgery) and duration of medication use (3.5±1.60 days and 3.3±0.97 days, respectively) between the Dexa and Furo groups (Table 2). The PSTS reached a peak level at 2.27±0.47 (2–3) days, 1.91±0.54 (1–3) days, and 1.38±0.74 (1–3) days after surgery in the control, Dexa, and Furo groups, respectively (Table 2). The peak time of PSTS in the Furo group was significantly earlier than that in the control (p<0.05) and Dexa (p<0.01) groups. Moreover, the peak time of PSTS in the Dexa group was not different from that in the control group.
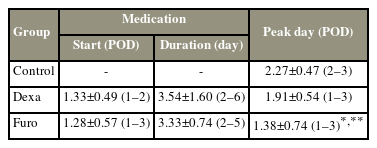
Starting day and duration of medication in the Dexa and Furo group, and the peak day (day with the thickest prevertebral soft tissue swelling) in the three groups
Daily changes in PSTS did not show a significant difference among the three groups preoperatively and 3 days postoperatively (Fig. 2). PSTS was significantly less in the Furo group than in the Dexa group on POD 4 (14.6±4.29 mm versus 18.7±4.21 mm, p<0.05), POD 5 (13.6±4.55 mm versus 18.1±3.79 mm, p<0.05), POD 6 (12.5±4.37 mm versus 17.3±3.09 mm, p<0.01), and POD 7 (12.0±4.77 mm versus 16.9±3.18 mm, p<0.05). PSTS became significantly smaller in the Furo group than in the control group on POD 5 (13.6±4.55 mm versus 16.7±1.43 mm, p<0.05), POD 6 (12.5±4.37 mm versus 16.4±1.39 mm, p<0.01), and POD 7 (12.0±4.77 mm versus 16.1±1.30 mm, p<0.01).
PSTS reductions on days 1–5 after the peak point (Peak+1, Peak+2, Peak+3, Peak+4, and Peak+5) were 0.5±0.50 mm, 0.4±0.75 mm, 0.9±0.85 mm, 1.9±0.94 mm, and 2.0±1.08 mm in the control group, 0.5±0.48 mm, 1.0±0.68 mm, 1.7±1.21 mm, 2.5±2.17 mm, and 2.8±2.26 mm in the Dexa group, and 3.0±2.09 mm, 4.0±2.39 mm, 5.3±3.21 mm, 6.0±3.18 mm, and 6.4±2.60 mm in the Furo group (Fig. 3). All PSTS reductions at the five checkpoints in the Furo group were significantly greater than those in the control (p<0.01) and Dexa (p<0.01) groups. However, no significant difference was found between the control and Dexa groups.
PSTS reductions on days 1–5 after starting the medication (Med+1, Med+2, Med+3, Med+4, and Med+5) were 0.1±0.68 mm, 0.4±1.18 mm, 0.9±1.38 mm, 1.7±2.18 mm, and 2.2±2.21 mm, respectively, in the Dexa group (Fig. 4). Those in the Furo group were 2.5±2.50 mm (p<0.001), 4.3±1.34 mm (p<0.001), 5.1±2.49 mm (p<0.01), 6.2±2.94 mm (p<0.01), and 6.9±3.06 mm (p<0.001), respectively, which indicated a significantly greater reduction than that in the Dexa group.
The VAS scores for the anterior neck discomfort on POD 7 were significantly different among the three groups (p=0.015). In the Furo group, the mean VAS on POD 7 was 1.9±1.25, which was significantly lower than those in the control and Dexa groups (p=0.029 and 0.045, respectively) (Table 3).
No noticeable hematoma was found at the surgery site. In any of the three groups, the PSTS did not need further interventions such as reoperation or intubation. No patients had mechanical instability or pseudoarthrosis. Dexamethasone or furosemide had no specific side effects on their respective groups. The intra- and inter-observer ICCs for PSTS (0.87 and 0.91, respectively) were within the acceptable range.
Discussion
Systemic intravenous steroids are widely used to reduce inflammation and related swelling. Short-term systemic steroids can cause side effects, such as hyperglycemia, high blood pressure, immunosuppression, and mood and sleep disturbances [13]. Older patients are more vulnerable to these side effects and at risk of surgical site infection. The effects of systemic steroid injection on PSTS remain controversial [10]. In multiple investigations, intravenous steroid therapy failed to establish definitive therapeutic benefits for PSTS [14,15]. Some studies have also reported the effect of the local placement of a steroid-soaked gelatin sponge after anterior cervical discectomy and fusion [16,17]. By contrast, furosemide is not associated with the aforementioned risk for side effects or infection. Furosemide is a loop diuretic that blocks the luminal Na-K-2Cl transporter in the thick ascending limb of the loop of Henle [18]. It is used for edema-related conditions, such as congestive heart failure, liver cirrhosis, and nephrotic syndrome [18,19]. Furosemide effectively treats soft tissue edema by reducing the extracellular fluid. If furosemide relieves PSTS after ACF surgery successfully, it could be a good substitute for steroids for PSTS management.
Song et al. [20] examined the efficacy of using simple lateral radiographs for evaluating PSTS after ACF. They found that the group with a PSTS >7.3 mm in the C3 area on the first two POD showed a higher rate of complications, such as dysphagia, dysphonia, and dyspnea, than the group with PSTS <7.3 mm (48.4% and 9.9%, respectively). The effects of dexamethasone and furosemide on the thickness of PSTS after ACF were compared using simple radiographs, as reported in a previous study [20], which is easy to apply clinically because of low cost and requirement of minimal effort. However, in the present study, symptom improvement did not correlate with PSTS improvement. Perhaps, the differences in symptom improvement will be found in larger studies. Among the total of 70 patients, 36 who used dexamethasone or furosemide had complications (51.4%), which was similar to the rates of previous studies [2,3].
In the present study, the peak swelling in the Furo group presented earlier than that in other groups, which appeared to be related to the faster and greater reduction in PSTS from the peak point or after the initiation of medication than that in the Dexa group. Therefore, intravenous furosemide therapy appears to be better than intravenous dexamethasone therapy in reducing PSTS.
In some patients in the Dexa group, the PSTS even increased after the administration of dexamethasone. For this reason, the standard deviation of the PSTS reduction after medication administration in the Dexa group was greater than that in the Furo group. This also supports the result that the peak PSTS in the Dexa group appeared later than in the Furo group.
A significant difference in the daily PSTS between the Furo and Dexa groups was noted from POD 4, and a significant difference between the Furo and control groups was observed from POD 5. This phenomenon does not mean that the use of dexamethasone is worse than that without medication administration. This may be because the Dexa group showed more severe PSTS on POD 1 than the control group owing to the different selection criteria between the two groups.
Considering that PSTS reduction after the medication administration was more significant in the Furo group than in the Dexa group, it can be assumed that PSTS results from soft tissue edema. Therefore, based on these data, we may expect a surgical site hematoma rather than soft tissue edema when the PSTS does not respond to furosemide, which needs additional research.
Kim et al. [21] assessed the steady decline in PSTS until 12 months, and Lee et al. [17] reported radiographic reduction until 2 weeks. In the present study, PSTS was measured for only 1 week after surgery; therefore, the PSTS values were still greater than the preoperative values. The measurement method and timing used in these two studies were different from those in the present study. Although a direct comparison is difficult, in accordance with the results of these studies, the PSTS in the present study would also show a continuous decrease after POD 7.
Furosemide is widely used for treating edematous conditions. Unlike steroids, the effects of diuretics are influenced by other factors related to the volume status, such as the function of the kidneys and the heart, and volume of intravenous intake of crystalloids [18,19,22]. Therefore, when furosemide is administered for PSTS reduction, the patient’s volume status should be assessed carefully. In the present study, since a relatively low dose of furosemide was used, furosemide had no side effects in relation to the patient’s volume status.
This study has several limitations. First, no correlation was found between radiologic and symptom improvements. Second, the study did not control the medical history of certain diseases that can influence the patient’s volume status. Third, selection bias could not be avoided because of the different selection criteria used for the medication groups and the control group. Furosemide and dexamethasone were used only for patients who complained of anterior neck discomfort after surgery. Consequently, the Furo and Dexa groups exhibited more severe swelling from the first day after the surgery than the control group. A randomized controlled study with computed tomography evaluation will strengthen our results. Fourth, this retrospective study has a relatively small sample size; thus, more extensive prospective studies are needed to confirm these results.
Conclusions
PSTS is a common complication of ACF that causes anterior neck discomfort, dysphagia, and dyspnea. Many surgeons have used intravenous dexamethasone therapy to reduce PSTS after ACF; however, it has side effects, and many controversies exist. In this study, intravenous furosemide therapy reduced PSTS effectively. Furosemide can be a good option for patients who are at risk of side effects of dexamethasone.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Writing–original draft: Ju-Sung Jang. Writing–review & editing: Young-Seok Lee, Myeong Jin Ko. Resources: Seong Hyun Wui, Kwang-Sup Song. Supervision: Seung Won Park. Final approval of the manuscript: all authors.