Delayed Neurological Deficits after Osteoporotic Vertebral Fractures: Clinical Outcomes after Surgery
Article information
Abstract
Study Design
Retrospective cohort.
Purpose
To review the clinical presentation of operated patients with delayed neurological deficits after osteoporotic vertebral fractures (OVFs).
Overview of Literature
Delayed neurological deficits can occur from 1 week to 5.7 months after OVFs. Baba has reported 78% good-to-excellent improvement (i.e., ≥50%) after 20 posterior (Cotrel-Dubousset) and 7 anterior (Kaneda in 4, Zielke ventral derotational spondylodesis in 2, and un-instrumented anterior fusion in 1) fusions. Predictive factors for neurological deficits include burst type, vacuum sign, kyphosis, angular instability, and retropulsion.
Methods
Patients with neurological deficits after OVF who received spinal operations between 2000 and 2016 were included.
Results
Totally, 28 patients with a mean age of 77 years underwent surgery. Neurological deficits occurred at an average of 5.4 weeks after the onset of back pain. The most common site was L1. Burst fracture was present in 14 patients and vacuum sign in seven. Surgery was performed within an average of 3.9 days of the onset of neurological deficit. Baba's score improved significantly from 5.96 to 9.81, with good-to-excellent improvement in 18 (64%) patients. Better outcomes based on Baba's scores (improvement>60% [median]) were associated with compression fractures, preoperative retropulsion of <41%, and correction of >16%. Poor improvement in Baba's scores (<25%) was associated with surgical complications and burst fracture type. Twenty-two patients (79%) regained walking ability, and seven of 15 (47%) patients demonstrated improved sphincter control at the latest follow-up. Six Frankel grade B patients did not achieve neurological recovery, four of whom exhibited postoperative surgical complications and died at 2 years because of medical problems. Implant migration occurred in six patients, albeit this was of no clinical significance.
Conclusions
Although OVFs are commonly considered benign, delayed neurological deficits can occur. The significant improvement in clinical function after surgery for neurological deficits is associated with compression (and not burst) fractures, lack of surgical complications, and optimal restoration of retropulsion.
Introduction
Osteoporosis and fragility fractures lead to major health problems among the elderly in Hong Kong. Approximately 37% of local postmenopausal women have T-scores of ≤−2.5 [1]. Spinal osteoporosis occurs among 45% of individuals aged 60–69 years and 56% of individuals aged >80 years [2]. Osteoporotic vertebral fractures (OVFs) occur among 16% and 33% of men and women aged >80 years, respectively [3]. Radiographic vertebral fractures occur among 5.0% and 12.1% of men and women, respectively [4].
The majority of OVFs heal after 8–12 weeks [5]. Surgery is indicated for neurological deficits, deformities, and incapacitating pain with conservative treatment failure. Moreover, 2% of in-hospital patients with OVFs (n=497) develop cord compression [6]. This study aimed to identify the clinical presentation and predictors of clinical outcomes among patients operated for delayed neurological deficits after OVFs.
Materials and Methods
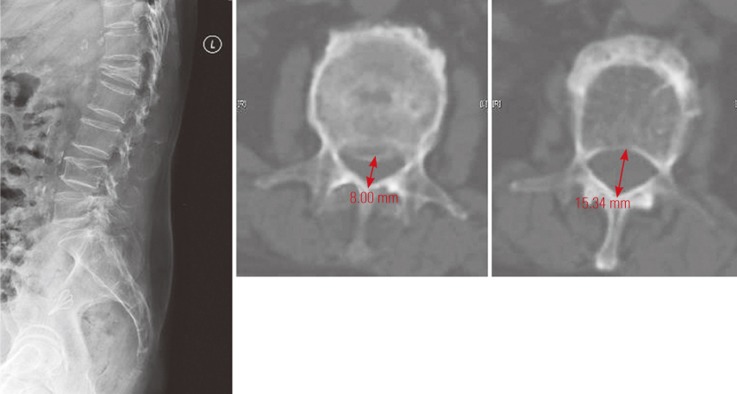
OVF patients aged >50 years who underwent surgery from 2000 to 2016 were identified using the Electronic Patient Record system. Patients with pathological fractures or injuries other than those due to falls from standing height were excluded. Pain and neurological status were charted according to Frankel and Medical Research Council (MRC) grading (Table 1) and Baba's scoring system (Table 2). Plain radiographs were evaluated to determine the type (compression or burst) and location of OVFs. The percentage loss of anterior vertebral height (LAH) and percentage loss of posterior vertebral height (LPH) were assessed as the loss of fractured vertebrae over adjacent vertebral heights. Mid-vertebral height was measured as the vertical distance between two endplates halfway between the anterior and posterior longitudinal line. The percentage of retropulsion was defined as the narrowest diameter of the spinal canal compared with adjacent cuts from transverse computed tomography images (Fig. 1). Nonparametric statistical analysis (i.e., median values for two-group comparison, Mann–Whitney U test for continuous variables, and Fisher's test for categorical variables) was performed considering the relative small sample size.
Results
The study population comprised 28 patients (21 women and seven men; mean age, 77 years [range, 55–88 years]). There was no history of injury in 16 (57%) patients. OVFs were diagnosed in patients with a history of low-energy-related fragility fractures and/or a positive bone densitometry (BMD) finding. Based on the lowest parameter obtained from the scans of both unoperated lumbar vertebrae and hips, postoperative dual-energy X-ray absorptiometry (DEXA) confirmed T-scores of ≤−2.5 in 13 of 16 patients (average, −3.3; range, −2.6 to −4.9), and the remaining three patients had T-scores of −1.5, −2.3, and −2.3, with a history of fragility fractures. During the early 2000s, patients were diagnosed using bedside calcaneal ultrasound. No documentation was available on the exact stiffness index or T-score values because the investigations were performed a long time ago. Sixteen patients had a history of vertebral fractures at other levels, seven had hip fractures, and two had distal radius fractures.
Fifteen (54%) patients were readmitted to the same orthopedic unit, with the first time admission for the new onset of back pain or fall injury and the second time admission for neurological deterioration. Another three patients received follow-up at the orthopedic clinic for the new onset of back pain before subsequent admission for neurological deficits. The remaining patients were either referred from other departments or OVF rehabilitation patients with a gradual deterioration in spinal neurology. The average time to onset of neurological deficits was 5.4 weeks (standard deviation [SD], 2.7 weeks [range, 1–12 weeks]).
Twenty-two (79%) patients could not walk, and six patients walked with heavy assistance. Fifteen (54%) patients had sphincter disturbance. Seven patients were classified as Frankel grade B, five as grade C, and 10 as grade D (Fig. 2). For fractures below the L2 level, the MRC grade for the most proximally involved myotomal level was 2 in two patients, 3 in two patients, and 4 in two patients. The mean preoperative Baba's score was 5.96 (SD, 2.43) [7]. Twenty fractures occurred at the thoracolumbar junction (T11–L1). The mean values of LAH and LPH were 63.0% and 28.7% postoperatively and 43.4% and 14.8% upon initial medical consultation (p=0.004 and 0.001), respectively. A significant decrease from 12.5 to 7.1 mm in the midvertebral height was observed. The average retropulsion was 36.5%. Vacuum sign was observed in seven patients and burst fractures in 14.

Frankel and MRC Grading of patients before and after operation. Pre-op, preoperative; post-op, postoperative; MRC, Medical Research Council.
The average time from the onset of neurological deficits to operation was 3.9 days (SD, 2.4 days; range, 1–8 days). Fifteen patients received anterior decompression followed by iliac crest/rib fusion, with nine patients receiving supplementary anterior spine instrumented fixation. Thirteen patients received posterior indirect decompression via ligamentotaxis, with 10 receiving cement augmentation and nine with instrumented fusion. Five levels of posterior fusion were performed in two patients with intraoperative findings of weak pedicle screw purchase. Immediate postoperative films demonstrated statistically significant mean improvement of the Cobb's angle to 8.3°; LAH, 36.3%; mid-vertebral height, 14.0 mm; and retropulsion, 20.5% (p=0.001, 0.001, <0.001, and <0.001, respectively) but not of LPH (p=0.06).
Postoperative infection occurred in four patients with poor improvement in Baba's scores. One patient who underwent anterior decompression developed a drain site infection that was treated with antibiotics alone; this patient died 2 years later because of infected sacral sores. The other three patients underwent posterior decompression: one had a dural tear that was intraoperatively repaired and developed superficial wound infection for which he underwent surgical debridement 3 weeks later; this patient died 1 year later because of pneumonia. Another patient underwent debridement at 1 week after initial operation and died 6 months later because of pneumonia. The remaining patient underwent implant removal 1 month later because of infection and developed progressive L1 kyphosis and translation. This patient remained chairbound with a permanent loss of sphincter function. According to Fisher's test, surgical complications were not correlated with the type of approach (p=0.311).
Six patients developed implant migration because of poor implant purchase. One patient died 3 months after surgery because of medical problems, and one patient remained chairbound because of the technical error of excessive posterior cage insertion, whereas the other four patients resumed walking mobility with a 50%, 60%, 67%, and 67% improvement in Baba's scores. No revision surgery was performed. No other in-hospital mortality occurred. One patient developed asymptomatic cement leakage after vertebroplasty. Implant complications were not correlated with the type (anterior, five cases and posterior, one case) of approach (p=0.173). Another four patients died because of unrelated medical problems at 7, 10, and 12 years after surgery.
The endpoint was defined as mortality or latest follow-up. Compared with immediate preoperative films, the final overall correction was statistically significant in terms of LAH, LPH, mid-vertebral height, and retropulsion (p=0.03, 0.04, 0.001, and 0.001, respectively) but not Cobb's angle (p=0.124).
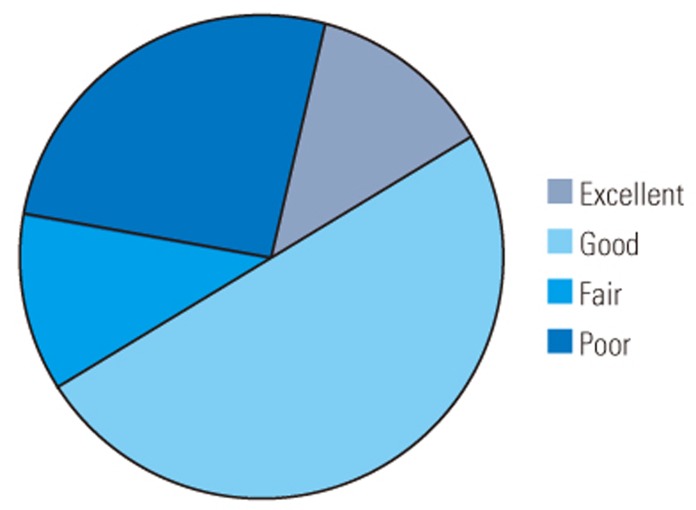
Excluding the two cases of mortality within 1 year of surgery and one case of recent surgery with only 9 months of follow-up, 25 patients received follow-up ranging from 1 to 9 years. Seventeen (61%) patients could walk unaided or with a stick and five with a frame; seven of 15 (47%) patients demonstrated improved sphincter control. Six patients with preoperative Frankel grade B lower limb function remained chairbound, and five of them required long-term Foley care. Five patients were classified as Frankel grade B (static), one as grade C (improved by one grade), eight as grade D (two static; five improved by one grade; one improved by two grades), and eight as grade E (all improved by one grade) (Fig. 2). The MRC grade of the most proximally involved myotomal level for lesions below L2 was 3 in one patient, 4+ in one patient, and 5 in four patients. The average Baba's scores improved from 5.96 to 9.81 at the latest follow-up (p=<0.001). In our series, the outcomes were rated as excellent among four patients, good among 14, fair among three, and poor among six (Table 2, Fig. 3).
The median improvement in Baba's score was 60% in our series, and analyses were performed to determine correlations of scores with demographic, operative, and radiological factors (Table 3). No correlation was found for demographic and operative factors. Statistical differences were observed for preoperative retropulsion (p=0.0404) and overall percentage of correction (p=0.004) between the group with less improvement in Baba's scores and that with more improvement in Baba's scores. Using the Fisher's test with cutoff median values of 41% for retropulsion and of 16% for correction, significant differences were observed between less and more improvement groups (p=0.024 and 0.004, respectively). Burst versus compression fractures were associated with less improvement of 37.1% (SD, 30.8%) versus 62.0% (SD, 18.2%) in Baba's scores (p=0.049) but not with the presence of vacuum sign (Baba's scores of 48.1% [SD, 33.8%] versus 51.1% [SD, 27.3%]). According to the results of the Fisher's test, poor improvement in Baba's scores (<25%) was correlated with postoperative surgical complications (p=0.001) and burst fractures (p=0.048) but not with implant migration (p=0.588).
Discussion
OVFs may present as neurological deficits due to spinal cord compression [8]. Approximately 58% (n=458) of OVFs occur at T11–L1 and 19% (n=151) at L2 [6]. Osteoporosis can be diagnosed clinically in addition to using T-score of ≤−2.5 for diagnosis. Under the direction of the National Bone Health Alliance, a clinical diagnosis of osteoporosis is defined as male or female patients aged >50 years who has had fragility fractures involving the hips, proximal humerus, spine, or wrist with or without a history of falls from a standing height [9]. In our study, 13 of 16 patients had T-score of ≤−2.5 according to DEXA scans. All patients had experienced either no fall or low-energy injuries. Associated fragility fractures occurred in 19 patients during their lifetimes. Before introducing DEXA in our locality, bedside calcaneal ultrasound was utilized for diagnosing osteoporosis. Calcaneal bone ultrasound has 89% sensitivity and 85% specificity [10] and has been the main tool used in developing Asian countries when DEXA scan with 90% sensitivity and specificity is not available [11].
A gradual or delayed onset of neurological deficits from 1 to 8 weeks [12] and 1 week to 3 months [13] after OVFs is common; one small study has reported an average of 5.7 months for onset in 164 patients [14]. In our locality, the introduction of personal safety alarm services in the homes of elderly patients has allowed easily access to ambulance and hospital services. The majority of our elderly patients are also receiving frequent medical attention in the public sector because of associated comorbidities. These factors combine to allow more timely and regular monitoring of patients' clinical status at the initiation of collapse, even in patients with a suboptimal mental status.
Middle column burst fractures were suggested to have a higher risk of neurological deficits because of retropulsed fragments [7]. In a Japanese series, approximately 91% of OVFs causing paraplegia were burst fractures (n=164) [14]. Angular instability of >15° and retropulsion of >42% were predictive factors for neurological deficits [15]. Our study reported similar findings of less favorable Baba's outcomes being associated with retropulsion of >41% and burst fracture type (defined as widening of the interpedicular distance by >3 mm on anteroposterior view) [16]. Although our study did not identify a relationship of the Cobb's angle or cleft sign with neurological outcomes, Cobb's angle of >30° was regarded as a precipitating factor in one study [7], and vacuum sign was observed in 22 patients before the onset of neurological deficits in another study (n=28) [17].
The surgical results were generally satisfactory, with substantial and, occasionally, complete neurological recovery. In Mori's review of 164 paraplegic patients, 129 were surgically treated by either anterior spinal fusion or posterior instrumentation, and 35 were conservatively treated; patients who were surgically treated showed better recovery of walking ability [14]. Saita et al. [18] has reported on 13 patients with a loss of walking ability who underwent posterior spinal shortening surgery, 10 of whom improved, two were static, and one had paralysis that deteriorated. In our series, 17 of 28 (61%) patients could walk unaided or with a stick at the endpoint following operation.
Frankel grading was used in many studies to document motor neurological deficits. In a series of 15 patients, one patient with Frankel grade A exhibited no recovery, whereas the remaining 14 patients with Frankel grades B–D exhibited improvement by two grades [19]. In another series, all 14 patients showed improvement from preoperative grades of C in seven and D in seven patients to postoperative grades of D in two and E in 11 patients [20]. In another series of 10 patients, the status of six patients improved by one grade, that if three remained unchanged, and that of one worsened by one grade [21]. In a series of eight patients with intravertebral cleft sign, the neurological status improved by at least one Frankel grade in seven (83%) patients with motor deficit [22]. Our study demonstrated motor recovery in terms of Frankel grading, with 15 (68%) of 22 patients showing improvement by at least one grade.
Sphincter dysfunction and recovery was seldom reported. In one series of 10 patients with paraplegia, only one of four patients with preoperative bowel and bladder incontinence had recovered functional control [21]. In another series of 13 patients with paraplegia, only one patient with urinary incontinence exhibited improvement in urinary function [18]. Our finding of sphincter function improvement in seven of 15 patients may be due to improved mobility and the treatment of urinary tract infection following surgery rather than an actual improvement in neurological function; this is a limitation of our study.
Two Japanese studies reported their outcomes using a pain–neurological scoring system modified from the Japanese Orthopaedic Association scoring system for lumbar spine surgery [7]. In their series of 2 20 posterior (Cotrel-Dubousset) and 7 anterior (Kaneda in 4, Zielke ventral derotational spondylodesis in 2, and un-instrumented anterior fusion in 1) fusions, Baba's scores improved from 8.3 preoperatively to 11.7 at follow-up, with an average improvement of 63%. In another series of 24 posterior Cotrel-Dubousset and 28 anterior titanium cage fusions, the average score improved from 7.6 to 11.6, with an average improvement of 63% [23].
Our study is limited by its retrospective nature, relatively small group of patients, lack of a control group and continuous BMD monitoring, and the diversity of surgeons. Further studies using bone mass monitoring, antiosteoporotic treatment, and advances in surgical technology need to be conducted for examining long-term radiological and clinical outcomes.
Conclusions
Although OVFs are common and generally considered benign, severe and delayed neurological deficits can occur following spinal cord compression. Improved clinical outcomes are associated with compression fractures, less initial retropulsion, lack of surgical complications, and an optimal restoration of retropulsion.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.