|
 |
- Search
| Asian Spine J > Volume 12(2); 2018 > Article |
|
Abstract
Purpose
To elucidate the prevalence of degenerative changes in the cervical and lumbar spine and estimate the degenerative changes in the cervical spine based on the degeneration of lumbar disc through a retrospective review of magnetic resonance (MR) images.
Overview of Literature
Over 50% of middle-aged adults show evidence of spinal degeneration. However, the relationship between degenerative changes in the cervical and lumbar spine has yet to be elucidated.
Methods
A retrospective review of positional MR images of 152 patients with symptoms related to cervical and lumbar spondylosis with or without a neurogenic component was conducted. The degree of intervertebral disc degeneration (IDD) was assessed on a grade of 1ŌĆō5 for each segment of the cervical and lumbar spine using MR T2-weighted sagittal images. The grades across all segments were summed to produce the degenerative disc score (DDS) for the cervical and lumbar spine. The patients were divided into two groups based on the IDD grade for each lumbar segment: normal (grades 1 and 2) and degenerative (grades 3ŌĆō5).
Results
DDSs for the cervical and lumbar spine were positively correlated. Significant differences in cervical DDSs between the groups were observed in all lumbar segments. Although there were no significant differences in cervical DDSs among the degenerative lumbar segment, cervical DDSs at the L1ŌĆō2 and L2ŌĆō3 segments tended to be higher than those at the L3ŌĆō4, L4ŌĆō5, and L5ŌĆōS degenerative segments.
Conclusions
Our study shows that participants with degenerative changes in the upper lumbar segments are more likely to have a certain amount of cervical spondylosis. This information could be used to lower the incidence of a missed diagnosis of cervical spine disorders in patients presenting with lumbar spine symptomology.
Degenerative skeletal disorders are common and serious problems worldwide, particularly in the aging populations. These disorders are classified as polygenic diseases because they are developed as a consequence of the interaction between an individual's genetic, lifestyle, and environmental factors. Degenerative disease of the spine is a common manifestation of the degenerative process, with the earliest spinal lesions thought to occur in the intervertebral discs. Intervertebral disc degeneration (IDD) typically appears in the second decade of life in men and in the third decade in women, with more than 50% of middle-aged adults showing some evidence of spinal spondylosis [1].
The human spine is subjected to large compressive preloads during activities of daily living. Depending on the position of the spine, the axial load exerted on the lumbar intervertebral discs can increase to magnitudes of up to three times the body weight. Age, individual somatotype, and environmental factors, such as activities of daily living and occupation, are the most important etiological factors of IDD [2]. Although not completely understood, genetic factors are increasingly being considered in the etiology of IDD. There is emerging evidence that gene polymorphisms in disc components, such as proteins, proteoglycans, cytokines, enzymes, and vitamin D receptor, play significant roles in the pathology of IDD, possibly by altering the normal homeostasis of the intervertebral disc and/or influencing the effects of environmental factors [34567].
Although IDD is common, clinical diagnosis and treatments are complicated because patients frequently present with complex neurogenic patterns of pain and impairment related to degenerative changes at multiple spinal levels. In these cases, assessment and treatment are often focused on the spinal region associated with the patient's primary complaint. For instance, a clinician may associate complaints of gait disturbances reported by patients with radiological evidence of lumbar spinal canal stenosis to cauda equina syndrome, focusing the neurological examination on the lower extremities, such as assessment of deep tendon reflexes, without considering the contribution of upper spinal lesions. An issue limiting the assessment and treatment in these cases is the absence of information regarding the relationship between degenerative changes at different levels of the spine.
Numerous studies investigating the degenerative processes of the human spine have reported the results of diagnostic imaging for the assessment and identification of spinal degeneration, as well as the resulting effects of the degeneration on the kinematics of the spine [891011121314151617]. However, to the best of our knowledge, few reports have so far investigated the correlation between degenerative changes of the cervical and lumbar spine. The aim of our study was to elucidate the prevalence of degenerative changes in the cervical and lumbar spine and estimate the degenerative changes in the cervical spine based on lumbar disc degeneration through a retrospective review of positional magnetic resonance imaging (MRI) scans.
This study included 152 patients (81 men and 71 women; age, 20ŌĆō62 years; mean age, 46.9 years) with symptoms related to cervical and lumbar spondylosis with or without a neurogenic component and who had not undergone prior spinal surgery.
This study was approved by the Institutional Review Board at University of Southern California Health Science Campus (IRB protocol no., HS-1400397).
All patients underwent positional cervical and lumbar MRI with a weight-bearing position on the same day. MRI was performed on a 0.6-T MRI scanner (Upright Multi-Position; Fonar Corp., New York, NY, USA) using a flexible surface coil. The imaging protocol included sagittal T1-weighted spin-echo sequences (repetition time [TR]/echo time [TE], 671/17 ms; slice thickness, 3.0 mm; field of view, 24 cm; matrix, 256├Ś200; and number of excitations [NEX], 2) and T2-weighted fast spin-echo sequences (TR/TE, 3,432/160 ms; slice thickness, 3.0 mm; field of view, 24 cm; and NEX, 2). All sequences were acquired without fat saturation.
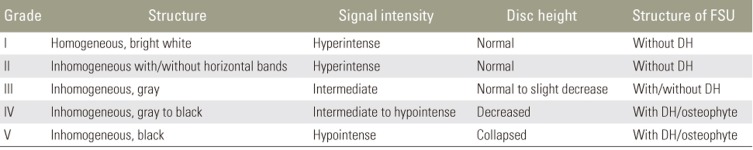
The patients' IDD was evaluated using a previously reported modified classification system based on the degenerative changes in the functional spinal unit [8910]. The classification system was used to establish an IDD grade, from grade 1, indicative of minimal evidence of IDD, to grade 5, indicative of substantive evidence of IDD. T2-weighted sagittal images of 1,520 intervertebral discs obtained from all the patients in this study were evaluated and classified using the five-grade system. The following intervertebral discs were evaluated: C2ŌĆō3, C3ŌĆō4, C4ŌĆō5, C5ŌĆō6, and C6ŌĆō7 for the cervical spine and L1ŌĆō2, L2ŌĆō3, L3ŌĆō4, L4ŌĆō5, and L5ŌĆōS for the lumbar spine. Patients were divided into two groups based on the IDD grade for each lumbar segment: normal (grades 1 and 2) and degenerative (grades 3ŌĆō5) intervertebral discs. Three independent observers were used to grade IDD, and their grades were compared to obtain the level of agreement. The grades attributed to each of the five intervertebral discs at the cervical and lumbar spine were summed to provide the respective degenerative disc score (DDS).
The computer software package StatMate III (ATMS Co. Ltd, Tokyo, Japan) was used. Pearson's correlation coefficient and MannŌĆōWhitney U-test were used for statistical analysis. A p-value of <0.05 was considered statistically significant. Kappa (╬║) value was calculated as a measure of the inter-observer reliability for IDD grading.
A high level of agreement (╬║=0.674) was noted among three independent observers who graded IDD. The distribution of IDD grades is reported in Table 1. The average disc grades for each segment and DDSs for the cervical and lumbar spine are shown in Tables 2 and 3, respectively. The highest IDD grade in the cervical spine was identified at C5ŌĆō6, followed by C6ŌĆō7 and C4ŌĆō5 segments. The IDD grade in the lumbar spine progressively increased from a minimum at the L1ŌĆō2 segment to more extensive evidence of degeneration in the lower lumbar segments.
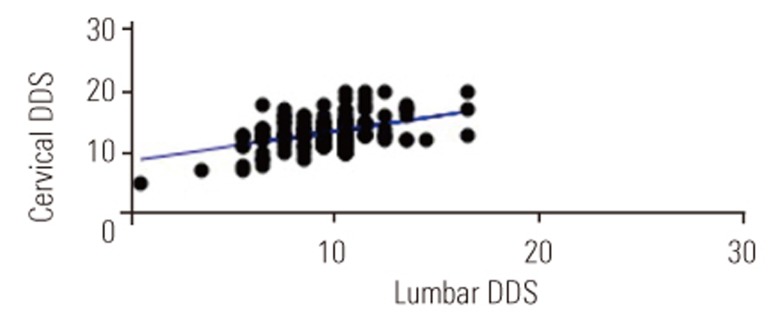
There were positive correlations between age and DDSs (cervical spine: p<0.01, r=0.25, r2=0.06; Fig. 1; lumbar spine: p<0.01, r=0.25, r2=0.06; Fig. 2). In addition, DDSs for the cervical and lumbar spine were positively correlated (p<0.001, r=0.44, r2=0.2) (Fig. 3).
The average cervical DDSs for each lumbar segment are shown in Table 4. We observed significant differences in cervical DDSs between the groups with normal and degenerative intervertebral disc in all lumbar segments. Although there were no significant differences in cervical DDSs in the degenerative lumbar segments, the cervical DDSs at the L1ŌĆō2 and L2ŌĆō3 segments tended to be higher than those at the L3ŌĆō4, L4ŌĆō5, and L5ŌĆōS degenerative segments.
The range of motion among the different spinal segments has largely been characterized based on functional plain radiographs obtained in non-weight-bearing positions. This provides evidence of a progressive, cephalad-to-caudal increase in the segmental range of motion, with greater magnitudes of motion in the lower segments of the lumbar spine, L4ŌĆō5 and L5ŌĆōS, compared with the upper lumbar segments [1213]. Weight-bearing alters the biomechanical state of intervertebral discs, with reported 5%ŌĆō15% decrease in the height and volume of lumbar spine discs in the weight-bearing compared with the non-weight-bearing positions [1415]. Therefore, imaging studies using non-weight-bearing positions may not accurately reflect the functional kinematics of the human spine. Using positional MRI to investigate the effects of degenerative changes in the lumbar intervertebral discs on the kinematics of the lumbar spine, Lee et al. [1617] reported a significantly larger range of motion at the L2ŌĆō3, L3ŌĆō4, and L4ŌĆō5 segments compared with the L1ŌĆō2 and L5ŌĆōS segments [111617]. Our results demonstrated that degenerative changes in the lumbar intervertebral discs are largest in the lower lumbar segments (L4ŌĆō5 and L5ŌĆōS segments), with lower evidence of degenerative changes in the upper lumbar segments (L1ŌĆō2 and L2ŌĆō3 segments). This lack of concordance between the range of segmental motion, weight-bearing disc volume, and IDD grade may be explained by the findings of Nachemson et al. [18] that the degree of degenerative changes in the lumbar segments does not correlate with the segmental range of motion. The progressive increase in degenerative changes from the upper to the lower segments of the lumbar spine agrees with our predicted pattern of IDD based on the weight-bearing function of the lumbar spine. In particular, the lower lumbar spine is the mechanical fulcrum between the spine and the pelvis. Therefore, the lower lumbar segments might be subjected to the highest magnitudes of mechanical stress during activities of daily living and, as a result, would also be the most influenced by somatotopic, genetic, and environmental factors.
In our study, age reflected the grade of degenerative changes in the cervical and lumbar spine, and there was a significant positive association of the degenerative changes between the cervical and lumbar spine. In particular, we found that participants with degenerative changes in the upper segments of the lumbar spine (L1ŌĆō2 or L2ŌĆō3 segments) were more likely to have a higher DDS of the cervical spine. Based on our study results, we propose the need to consider the presence of cervical spine disorders in patients being evaluated and treated for lumbar spine disorders. A prognostic determination of degenerative changes in the cervical spine based on the assessment of IDD of the spinal segments in the upper lumbar spine could lower the incidence of missed diagnosis of cervical spine disorders in patients presenting with lumbar spine symptomology.
The limitations of our study need to be considered in the application of its outcome to practice. Foremost, only IDD in the cervical and lumbar spine was assessed, without consideration of neurological symptoms and impairments, such as the degree of spinal cord or cauda equina entrapment. Therefore, our results proof-of-principle data to support the development of a larger, population-based study to establish the prevalence of degenerative changes for all segments of the spine, as well as to clarify the relationships between the degenerative changes at multiple spinal levels.
Our study showed that patients with degenerative changes in the upper lumbar segments are more likely to have certain degree of cervical spondylosis. This information could lower the incidence of missed diagnosis of cervical spine disorders in patients presenting with lumbar spine symptomology.
Acknowledgments
The study was supported by departmental funds. Authors would like to thank AiM Radiology Medical Group, especially to Yusuf A. Khan, Sameer U. Khan and Aziza Qadir MD for their help on obtaining and uploading kMRI images into the database.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Disclosures outside of submitted work: ZB-Xenco Medical (consultancy), AO Spine (consultancy); JCWŌĆōRoyaltiesŌĆōBiomet, Seaspine, Amedica, Synthes; investments/options: Bone Biologics, Expanding Ortho, Pearldiver, Flexuspine, Fziomed, Benvenue, Promethean, Nexgen, Electrocore, Surgitech; board of directors: North American Spine Society, AO Foundation, Cervical Spine Research Society; editorial boards: Spine, JAAOS, The Spine Journal, Clinical Spine Surgery, Global Spine Journal; and fellowship funding (paid to institution): AO Foundation.
References
1. Zigler JE, Strausser DW,The aging spine. Fardon DF, Garfin SR, editors. Orthopaedic knowledge update: spine 2. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2002. p.123ŌĆō133.
2. Avinash GP, Ioannis NG, Leonald IV,Biomechanics of the spine. Spivak JM, Connolly PJ, editors. Orthopaedic knowledge update: spine 3. Rosemont (IL): American Academy of Orthopaedic Surgeons; 2006. p.25ŌĆō32.
3. Hanaei S, Abdollahzade S, Khoshnevisan A, Kepler CK, Rezaei N. Genetic aspects of intervertebral disc degeneration. Rev Neurosci 2015 26:581ŌĆō606. PMID: 25996483.



4. Eskola PJ, Mannikko M, Samartzis D, Karppinen J. Genome-wide association studies of lumbar disc degeneration: are we there yet? Spine J 2014 14:479ŌĆō482. PMID: 24210639.


5. Nakki A, Battie MC, Kaprio J. Genetics of disc-related disorders: current findings and lessons from other complex diseases. Eur Spine J 2014 23(Suppl 3): S354ŌĆōS363. PMID: 23838702.


6. Battie MC, Lazary A, Fairbank J, et al. Disc degeneration-related clinical phenotypes. Eur Spine J 2014 23(Suppl 3): S305ŌĆōS314. PMID: 23884550.


7. Ikegawa S. The genetics of common degenerative skeletal disorders: osteoarthritis and degenerative disc disease. Annu Rev Genomics Hum Genet 2013 14:245ŌĆō256. PMID: 24003854.


8. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001 26:1873ŌĆō1878. PMID: 11568697.


9. Miyazaki M, Hong SW, Yoon SH, et al. Kinematic analysis of the relationship between the grade of disc degeneration and motion unit of the cervical spine. Spine (Phila Pa 1976) 2008 33:187ŌĆō193. PMID: 18197105.


10. Morishita Y, Hida S, Miyazaki M, et al. The effects of the degenerative changes in the functional spinal unit on the kinematics of the cervical spine. Spine (Phila Pa 1976) 2008 33:E178ŌĆōE182. PMID: 18344847.


11. Tan Y, Aghdasi BG, Montgomery SR, Inoue H, Lu C, Wang JC. Kinetic magnetic resonance imaging analysis of lumbar segmental mobility in patients without significant spondylosis. Eur Spine J 2012 21:2673ŌĆō2679. PMID: 22674194.



12. Hayes MA, Howard TC, Gruel CR, Kopta JA. Roentgenographic evaluation of lumbar spine flexion-extension in asymptomatic individuals. Spine (Phila Pa 1976) 1989 14:327ŌĆō331. PMID: 2711247.


13. Pearcy M, Portek I, Shepherd J. Three-dimensional x-ray analysis of normal movement in the lumbar spine. Spine (Phila Pa 1976) 1984 9:294ŌĆō297. PMID: 6374922.


14. Malko JA, Hutton WC, Fajman WA. An in vivo magnetic resonance imaging study of changes in the volume (and fluid content) of the lumbar intervertebral discs during a simulated diurnal load cycle. Spine (Phila Pa 1976) 1999 24:1015ŌĆō1022. PMID: 10332795.


15. Botsford DJ, Esses SI, Ogilvie-Harris DJ. In vivo diurnal variation in intervertebral disc volume and morphology. Spine (Phila Pa 1976) 1994 19:935ŌĆō940. PMID: 8009352.


16. Lee SH, Daffner SD, Wang JC, Davis BC, Alanay A, Kim JS. The change of whole lumbar segmental motion according to the mobility of degenerated disc in the lower lumbar spine: a kinetic MRI study. Eur Spine J 2015 24:1893ŌĆō1900. PMID: 24676853.


17. Lee SH, Daffner SD, Wang JC. Does lumbar disk degeneration increase segmental mobility in vivo?: segmental motion analysis of the whole lumbar spine using kinetic MRI. J Spinal Disord Tech 2014 27:111ŌĆō116. PMID: 24795947.


18. Nachemson AL, Schultz AB, Berkson MH. Mechanical properties of human lumbar spine motion segments: influence of age, sex, disc level, and degeneration. Spine (Phila Pa 1976) 1979 4:1ŌĆō8. PMID: 432710.


Fig.┬Ā1
A significant correlation between age and DDS for the cervical spine; Pearson's correlation coefficient (p<0.01, r=0.25, r2=0.06). DDS, disc degenerative score.
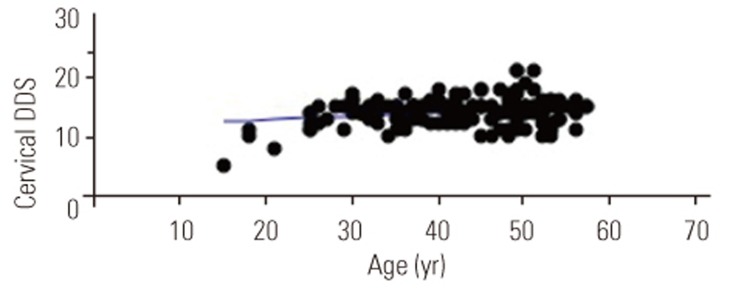
Fig.┬Ā2
A significant correlation between age and DDS for the lumbar spine; Pearson's correlation coefficient (p<0.01, r=0.25, r2=0.06). DDS, disc degenerative score.
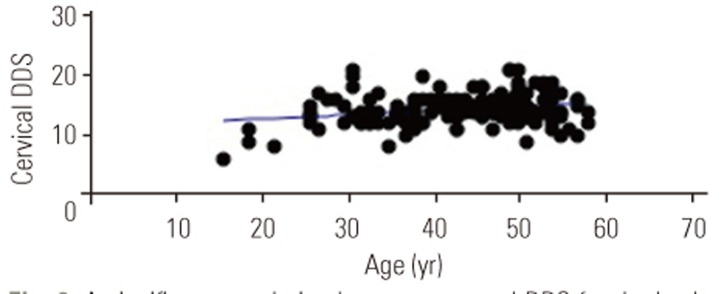
Fig.┬Ā3
A significant correlation between DDSs for the cervical and lumbar spine; Pearson's correlation coefficient (p<0.001, r=0.44, r2=0.2). DDS, disc degenerative score.