 |
 |
- Search
| Asian Spine J > Volume 13(4); 2019 > Article |
|
Abstract
Purpose
This study aims to evaluate the safety and efficacy of bone morphogenetic protein-2 (BMP-2) in transforaminal lumbar interbody fusion (TLIF) with regard to postoperative radiculitis.
Overview of Literature
Bone morphogenetic protein (BMP) is being used increasingly as an alternative to iliac crest autograft in spinal arthrodesis. Recently, the use of BMP in TLIF has been examined, but concerns exist that the placement of BMP close to the nerve roots may cause postoperative radiculitis. Furthermore, prospective studies regarding the use of BMP in TLIF are lacking.
Methods
This prospective study included 77 patients. The use of BMP-2 was determined individually, and demographic and operative characteristics were recorded. Leg pain was assessed using the Visual Analog Scale (VAS) for pain and the Sciatica Bothersome Index (SBI) with several secondary outcome measures. The outcome data were collected at each follow-up visit.
Results
Among the 77 patients, 29 were administered with BMP. Postoperative leg pain significantly improved according to VAS leg and SBI scores for the entire cohort, and no clinically significant differences were observed between the BMP and control groups. The VAS back, Oswestry Disability Index, and Short-Form 36 scores also significantly improved. A significantly increased 6-month fusion rate was noted in the BMP group (82.8% vs. 55.3%), but no significant differences in fusion rate were observed at the 12- and 24-month follow-up. Heterotopic ossification was observed in seven patients: six patients and one patient in the BMP and control groups, respectively (20.7% vs. 2.1%). However, no clinical effect was observed.
Conclusions
In this prospective observational trial, the use of BMP in TLIF did not lead to significant postoperative radiculitis, as measured by VAS leg and SBI scores. Back pain and other functional outcome scores also improved, and no differences existed between the BMP and control groups. The careful use of BMP in TLIF appears to be both safe and effective.
Recombinant human bone morphogenetic proteins (BMPs) are osteoinductive and are increasingly used as alternatives to iliac crest bone grafting for spinal arthrodesis. Although several different BMPs have been identified, only bone morphogenetic protein-2 (BMP-2) is approved by the Food and Drug Administration for use in anterior lumbar interbody fusion (ALIF) [1]. The physician-directed use of BMP-2 in other interbody fusions, including posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF), has been described [2,3]. Regardless of the technique, the use of BMP-2 in ALIF, PLIF, and TLIF have shown high fusion rates of >90% [4]. Given the increasing use of BMP-2 for other clinically directed indications, the need to define BMP-2-related complications is becoming increasingly important.
Previous studies have shown that lumbar interbody arthrodesis can be performed safely and effectively by using TLIF, which affords a posterior-only technique without extensive dural retraction [5-7]. With the increasing popularity of the TLIF approach, surgeons have begun using BMP-2 as an alternative to bone graft in interbody implants [3,8-10]. Previous studies have shown improved fusion rates with the use of BMP-2 compared with local bone graft in TLIF [10,11]. Furthermore, the use of BMP-2 avoids the need to obtain an iliac crest bone graft, which can be associated with donor-site morbidity [4]. Despite these advantages, postoperative complications, including seroma formation, vertebral osteolysis, and postoperative radiculitis, have been reported after BMP-2 use in TLIF, thus prompting some researchers to caution against its use [3,8,12,13]. Postoperative radiculitis rates as high as 16.7% have been reported in the literature [12]. This has been hypothesized to stem from an inflammatory reaction caused by the BMP-2 in close proximity to nerve roots [14]. Furthermore, other studies have shown increased heterotopic ossification after BMP use and bony extension into the spinal canal or foramina, which could possibly cause nerve root irritation [11,15-17]. However, previous studies that examined this effect have been limited to retrospective case series.
This prospective observational study aimed to evaluate the safety and efficacy of BMP-2 use via a TLIF approach and to examine the incidence of postoperative radiculitis.
The prospective observational study was conducted at a Mayo Clinic, Rochester, MN after being approved by the Institutional Review Board (Identification no., 08-00802). Part of the approval was contingent on a detailed informed consent of each patient enrolled in the study regarding the risks, benefits, and alternatives to study participation. The Visual Analog Scale (VAS) and Sciatica Bothersome Index (SBI) scores were used to compare outcomes in patients undergoing TLIF with BMP compared with those in patients undergoing other bone grafting techniques only. The secondary outcomes included radiographic results, fusion rates, and other clinical outcome scores following TLIF, including VAS back, Oswestry Disability Index (ODI), and Short-Form 36 (SF-36) scores.
The inclusion criteria for this study were patients between 18 and 75 years with lumbar degenerative conditions (e.g., degenerative disk diseases and spondylolisthesis) who presented with significant leg complaints after >6 weeks of unsuccessful conservative treatment. Patients who were considered good candidates for TLIF were offered enrollment. The exclusion criteria included interbody fusions performed at levels other than between L1 and S1, TLIFs performed at more than three levels, history of previous fusion attempt of the involved levels, morbid obesity (body mass index >40 kg/m2), or surgery performed for nondegenerative indications. Informed consent was obtained from all study subjects.
The patients enrolled in the study underwent TLIF with either BMP-2 and bone graft or bone grafting only. The choice of BMP-2 use was determined individually by the surgeon and patient with relevant patient-specific factors favoring the use of BMP-2, including poor bone quality, desire to avoid donor-site morbidity associated with iliac crest bone harvesting, and insurance coverage of BMP-2. All patients underwent standard posterior lumbar instrumented arthrodesis with bilateral pedicle screw fixation. Surgeons performed the surgery with their choice of instrumentation and TLIF cage. Minimal access approaches were used in 12 cases. The BMP-2 product used was Infuse (Medtronic, Minneapolis, MN, USA). Individual BMP-2-soaked collagen sponges were inserted into TLIF cages, and the remaining sponges were placed posteriorly over the exposed transverse processes. The size of the Infuse kit was primarily determined intraoperatively on the basis of the number of levels to be fused. BMP-2 dosages ranged from 0.04 to 0.11 mg/kg with a mean of 0.06 mg/kg.
The standard follow-up was performed at 6 weeks, 3 months, 6 months, 1 year, and 2 years. VAS leg, VAS back, SBI, ODI, and SF-36 scores were collected at each follow-up visit. Furthermore, postoperative radiculitis was carefully evaluated by the surgeon at follow-up. Any new symptoms or objective findings of radiculitis were recorded.
Fusion was assessed using radiographs and computed tomographic scans at 6 months, 1 year, and 2 years according to the preference of the surgeon. This was defined as bridging bone between adjacent vertebral bodies either through or around the implant, <5° of angular motion, and <3 mm of translation. Imaging was also assessed for subsidence, which is defined as cage settling ≥3 mm. Heterotopic ossification was also assessed on imaging for either canal or foraminal encroachment.
The mean for continuous outcomes and frequency (%) for categorical outcomes were assessed. The two-group comparisons were assessed between baseline outcomes and each follow-up period with two-sample t-test and Fisher’s exact test for continuous and categorical variables, respectively. Analysis was used to assess the changes in outcomes over time. Statistical significance was considered for p<0.05. All analyses were performed using JMP software for Windows ver. 9.0.1 (SAS Institute Inc., Cary, NC, USA).
From May 2009 to September 2013, 77 patients were enrolled in the study. BMP and bone grafting were used in 29 and 48 patients, respectively. Although the patients in the control group were 6 years older on average than the patients in the BMP group (Table 1), sex, diabetes, and tobacco use were not significantly different between the groups. The control and BMP groups used 29 (60.4%) and 1 (3.5%) allograft spacer, respectively. Blood loss and use of iliac crest autograft were significantly higher in the control group at 1,035.2 versus 608.6 mL and 41.7% versus 6.9%, respectively (Table 2).
No unanticipated BMP-related adverse events were identified by the surgeons during the study, including no clinical documentation of postoperative radiculitis.
Leg pain was not significantly different preoperatively as measured by VAS leg scores (Table 3, Fig. 1). Both groups showed significant improvements in leg pain postoperatively compared with the preoperative scores (−3.9 and −3.2 for the BMP and control groups at 6 weeks, respectively; p<0.001). The magnitude of improvement was not significantly different between the two groups (p=0.289). The improvement in postoperative leg pain was significantly maintained during follow-up for both groups up to 2 years (Fig. 1).
SBI scores demonstrated a similar relationship to VAS leg scores for both groups (Table 4, Fig. 2). The SBI scores between the BMP and control groups were not significantly different preoperatively. At 6 weeks follow-up, a significant improvement was noted in SBI scores (−2.12 and −1.86 for the BMP and control groups, respectively; p<0.001). The magnitude of improvement was not significantly different between the two groups (p=0.804). The improvement in SBI scores were significantly maintained during the follow-up period for both groups up to 2 years.
The preoperative back pain measured by VAS scores was not significantly different between the BMP and control groups (Table 5, Fig. 3). At 6 weeks, a significant improvement of −2.6 and −2.9 in back scores was noted for the BMP and control groups, respectively (p<0.001). No significant differences were noted in the magnitude of improvement between the two groups (p=0.214). The improvement in VAS back scores were significantly maintained over the 2-year follow-up period.
ODI scores were used to assess functional pain postoperatively (Table 6, Fig. 4). At all follow-up intervals, both groups had significant improvements in ODI scores. No significant differences were found between the groups with respect to improvement at 6 weeks, 3 months, 6 months, 1 year, and 2 years (p=0.638, 0.128, 0.174, 0.085, and 0.125, respectively).
SF-36 outcomes were broken down into physical component scores (PCSs) and mental component scores (MCSs) to assess general health. Significant improvements in both PCS and MCS were noted in the BMP and control groups out to 2 years, with no significant differences between the groups at 2 years (Tables 7, 8 and Figs. 5, 6).
At 6 months, the fusion rate in the BMP group was significantly higher than that in the control group (82.8% versus 55.3%, p=0.024). No significant difference was found between the BMP and control groups at the 1-year follow-up (100% versus 86.8%, p=0.147) and 2-year follow-up (100% versus 90.9%, p=0.466) (Table 9, Fig. 7). Figs. 8 and 9 show successful fusions in non-BMP and BMP patients, respectively. Heterotopic ossification was seen in six patients in the BMP group (20.7%) compared with one patient in the control group (2.1%, p=0.013). None of the patients with heterotopic ossification had any resulting clinical symptoms, including symptoms of recurrent leg pain. Cage subsidence was observed in three patients in the control group (6.3%) without any noted postoperative symptoms (Table 10).
Four patients underwent reoperation (two patients each in the BMP and control groups). In the BMP group, one patient underwent reoperation for a misplaced screw 1 day postoperatively, and one patient underwent revision decompression at 8 months. Revision decompression was performed following an L4–L5 TLIF for bilateral lower extremity pain. The patient continued to experience residual symptoms in the right lower extremity secondary to a right-sided L5–S1 lateral recess stenosis that was not addressed at the index surgery. No heterotopic ossification was found on preoperative CT or was identified during revision surgery. The patient had immediate relief of these symptoms following L5–S1 lateral recess decompression. In the control group, one patient underwent reoperation for screw pullout 2 months postoperatively, and one patient underwent hematoma evacuation 6 days postoperatively.
The use of BMP-2 in TLIF has several potential advantages. BMP-2 allows for high interbody fusion rates while avoiding the increased morbidity associated with iliac crest autograft [18-20]. Previous studies have shown that >50% of patients with iliac crest autograft may complain of donor-site pain as long as 6 months postoperatively [4]. Given that efforts should be exerted to improve patient outcomes following spinal fusion, establishing the safety and efficacy of BMP use is important [2].
In a series of 48 patients who underwent single-level TLIF with BMP-2, Rihn et al. [12] reported a 16.7% incident of transient radiculitis. Although this value is the highest incidence reported in the literature, Owens et al. [3] studied a larger series of patients undergoing TLIF with BMP-2 and found a 6.4% rate of new or worsened radiculopathy, 2.5% which were attributed to foraminal seroma formation secondary to BMP-2 use. However, postoperative radiculitis can occur following TLIF without BMP-2. In a series of 100 consecutive patients who underwent TLIF without BMP, Potter et al. [21] reported a 7% rate of transient radiculopathy, thus suggesting that technical factors, such as nerve root retraction and injury during disk preparation and cage insertion, may play a role in instigating radiculopathy. Additional techniques may reduce or eliminate the incidence of BMP-2. For instance, some have suggested that BMP-2 may have an inflammatory effect when nerve roots are directly exposed, and Rihn et al. [22] found a reduction in postoperative radiculitis from 20.4% to 5.4% with the use of a hydrogel sealant. This finding suggests that radiculitis rates may be further reduced with TLIF techniques that minimize exposure and neural elements. Other authors have shown that no BMP-2-related neurologic complications occurred when structural allografts were used to close off the disc space, thereby preventing BMP-2 contact with the neural elements [8].
Despite a sample size that is commensurate with prior studies, we did not observe any instances of BMP-related postoperative radiculitis. The measurements of VAS leg and SBI scores showed a significant improvement in both groups, with no differences in the magnitude of scores between the groups. These findings were consistent with the prospective study by Haid et al. [10], who examined the outcomes after BMP use in PLIF and found a significant improvement in VAS leg scores for both the BMP and control groups.
The use of BMP-2 in our study led to heterotopic ossification at 20.7%. This is consistent with the prospective analysis by Joseph and Rampersaud [11], who examined heterotopic ossification following minimal access PLIF and TLIF with and without BMP. In their study, the heterotopic ossification rate was 20.8% and 8.3% in the BMP and control groups, respectively. The rate of extradiscal heterotopic ossification can be as high as 75% following BMP use in lumbar interbody fusions [10]. However, other studies have shown that heterotopic bone formation did not occur following BMP-2 use in TLIF, thus suggesting that certain technical factors may play a role in the rate [4,8,23]. The clinical outcomes for patients with heterotopic ossification vary in the literature. Haid et al. [10] and Joseph and Rampersaud [11] found that no clinical sequelae were associated with heterotopic ossification, and this result was consistent with our study. However, other authors have reported complications that are related to postoperative neuroforaminal heterotopic ossification [15,24]. Singh et al. [24] reported a 1.7% rate of postoperative radiculopathy following BMP-2 use in 573 minimally invasive TLIF procedures. Furthermore, all cases of postoperative radiculopathy demonstrated neuroforaminal bony overgrowth that required reoperation.
Vertebral osteolysis and cage subsidence have been described as potential complications following BMP-2 use in interbody fusions [3]. Osteolysis rates vary widely in the literature from 0.5% to 82% in some series [3,25-27]. Osteolysis or cage subsidence was not observed in our BMP-2 treated group. Although the cause of vertebral osteolysis is unclear, in vivo studies have shown increased osteoclastic activity with high concentrations of BMP-2 in a cancellous bone environment [28]. Therefore, technical factors, such as BMP-2 dosing or endplate violations, may play a role in the development of osteolysis and subsidence.
The patients in both the BMP and control groups in our study had reduced preoperative back pain, which was consistent with previous studies examining interbody fusions [5,29], and a concordant improvement in ODI and SF-36 scores. Similar to the study of Burkus et al. [1], who examined BMP-2 use in ALIF, improvements in clinical and functional outcomes were not significantly different between the BMP and control groups.
Although short-term fusion rates were higher in the BMP group at 6 months (82.8% versus 55.3%, p=0.024), no significant differences between the groups were observed at 1- and 2-year follow-up. These findings were consistent with those of Joseph and Rampersaud [11] with regard to the short-term fusion rates following BMP-2 use in TLIF. They found a significantly higher fusion rate with BMP-2 use at 6 months (91.3% versus 50%). This finding suggests that although the osteoinductive properties of BMP-2 greatly accelerate the fusion process, techniques with bone grafting alone may eventually achieve similar fusion rates. These high fusion rates at long-term follow-up are consistent with previous studies on ALIF, PLIF, and TLIF that showed fusion rates of ≥90% regardless of adjunctive technique [4,8,10,11,19,30]. Despite similar long-term fusion rates, blood loss was significantly higher in the non-BMP group possibly because nearly half of patients received iliac crest autograft. As others have suggested, blood loss morbidity associated with iliac crest autograft harvest makes BMP-2 an attractive alternative in TLIF [4].
Our study has some limitations. Despite the prospective nature of this study, it was neither randomized nor blinded, thus possibly introducing some selection and observational bias. Although our sample size was similar to previous studies, it may still have been underpowered to fully avoid a type II error. Furthermore, the instances of transient radiculitis occurring between the time of discharge and the first postoperative visit at 6 weeks may have been missed given the study design.
In conclusion, the use of BMP-2 in TLIF in our series did not lead to significant postoperative radiculitis as measured by VAS leg and SBI scores. BMP-2 use was associated with significantly increased rates of heterotopic ossification. However, this did not affect the clinical outcomes. Furthermore, we observed a statistically significant improvement in fusion rates with the use of BMP-2 at 6 months, although the fusion rates after 1 year was not different. The careful use of BMP-2 in TLIF seemed to be a safe and effective alternative to conventional bone grafting techniques.
Fig. 1.
VAS leg scores of the BMP group versus the control group at baseline and follow-up. VAS, Visual Analog Scale; BMP, bone morphogenetic protein.
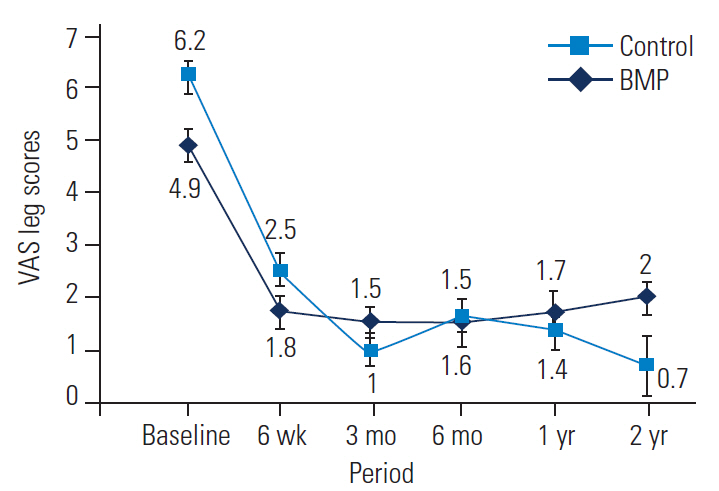
Fig. 2.
SBI scores of the BMP group versus the control group at baseline and follow-up. SBI, Sciatica Bothersome Index; BMP, bone morphogenetic protein.

Fig. 3.
VAS back scores of the BMP group versus the control group at baseline and follow-up. VAS, Visual Analog Scale; BMP, bone morphogenetic protein.
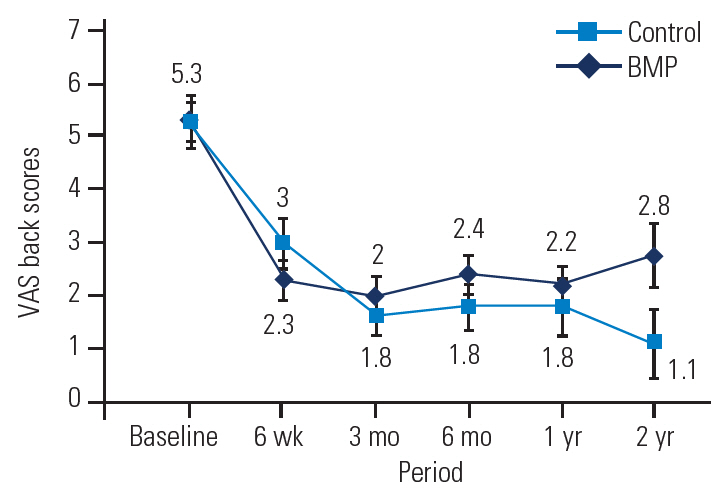
Fig. 4.
ODI scores of the BMP group versus the control group at baseline and follow-up. BMP, bone morphogenetic protein. ODI, Oswestry Disability Index.
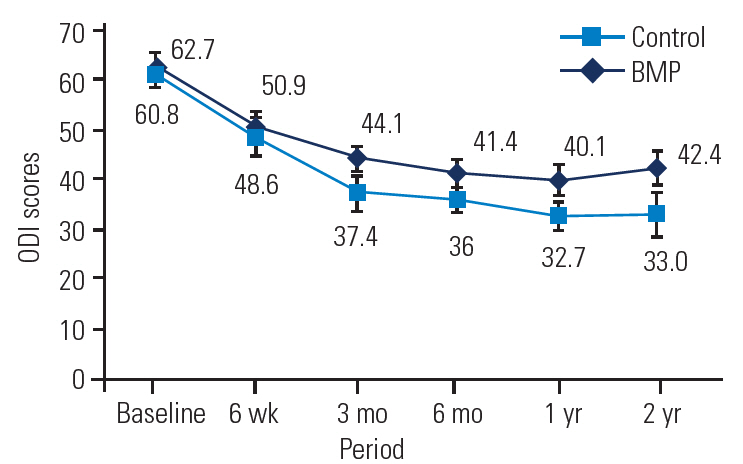
Fig. 5.
SF-36 PCS of the BMP group versus the control group at baseline and follow-up. SF-36, Short-Form 36; PCS, physical component scores; BMP, bone morphogenetic protein.
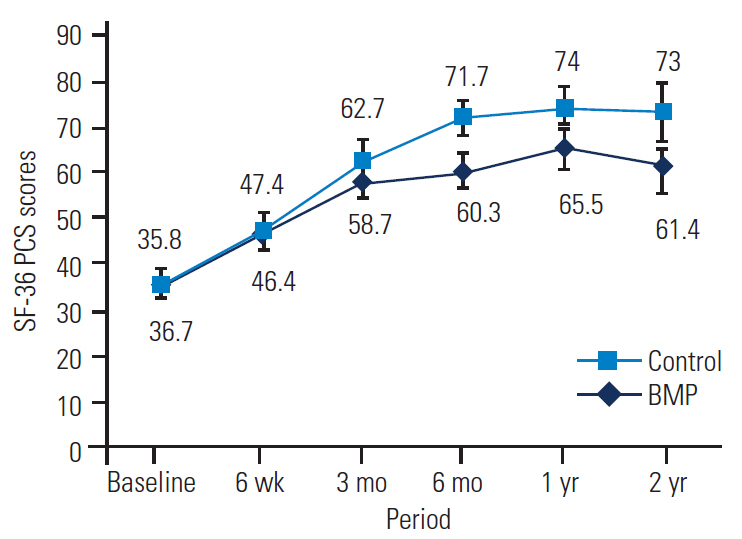
Fig. 6.
SF-36 MCS of the BMP group versus the control group at baseline and follow-up. SF-36, Short-Form 36; MCS, mental component scores; BMP, bone morphogenetic protein.
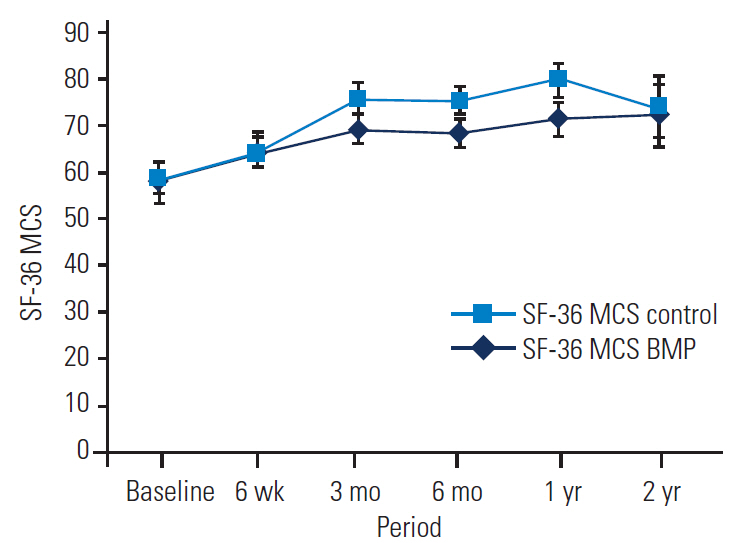
Fig. 7.
Fusion rates of the BMP group versus the control group at 6, 12, and 24 months. BMP, bone morphogenetic protein. a)Indicates a significant difference.

Fig. 8.
Sagittal computed tomography of a 46-year-old patient showing solid fusion across L4–L5 transforaminal lumbar interbody fusion not utilizing bone morphogenetic protein at 1 year postoperatively.

Fig. 9.
Sagittal computed tomography of a 66-year-old patient showing solid fusion across L4–L5 transforaminal lumbar interbody fusion utilizing bone morphogenetic protein at 1 year postoperatively.
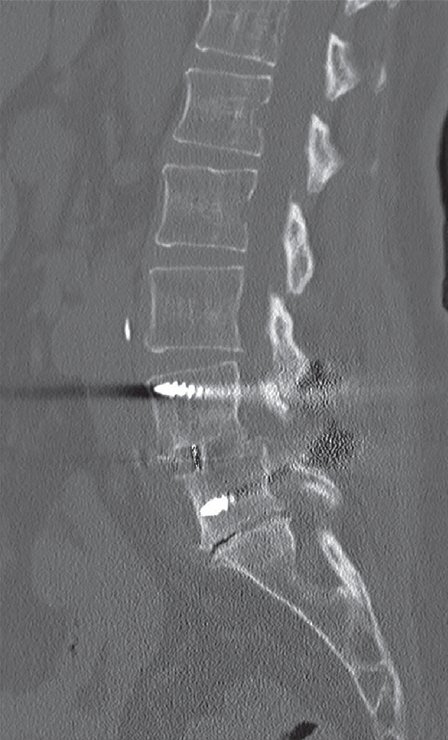
Table 1.
Patient demographic data
Table 2.
Operative data
Table 3.
Leg pain (Visual Analog Scale leg)
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 6.2±2.2 | 48 | 4.9±2.7 | ||
| 6 wk | 27 | 2.5±2.8 | 0.289 | 34 | 1.8±2.3 | |
| Improvement from preop | -3.9 | <0.001a) | -3.2 | <0.001a) | ||
| 3 mo | 21 | 1.1±1.4 | 0.303 | 37 | 1.5±2.0 | |
| Improvement from preop | -4.9 | <0.001a) | -3.2 | <0.001a) | ||
| 6 mo | 23 | 1.6±2.2 | 0.898 | 38 | 1.5±2.1 | |
| Improvement from preop | -4.8 | <0.001a) | -3.7 | <0.001a) | ||
| 12 mo | 18 | 1.4±2.1 | 0.645 | 32 | 1.7±2.2 | |
| Improvement from preop | -4.9 | <0.001a) | -3.1 | <0.001a) | ||
| 24 mo | 12 | 0.7±1.0 | 0.049a) | 21 | 2.0±2.8 | |
| Improvement from preop | -4.8 | <0.001a) | -2.8 | <0.001a) | ||
Table 4.
Sciatic Bothersome Index Scores
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 4.6±1.1 | 48 | 4.2±1.2 | ||
| 6 wk | 27 | 2.5±1.3 | 0.804a) | 34 | 2.4±1.6 | |
| Improvement from preop | -2.12 | <0.001b) | -1.86 | <0.001b) | ||
| 3 mo | 21 | 2.0±1.2 | 0.404a) | 37 | 2.3±1.5 | |
| Improvement from preop | -2.8 | <0.001b) | -1.9 | <0.001b) | ||
| 6 mo | 23 | 1.8±1.1 | 0.208a) | 40 | 2.2±1.7 | |
| Improvement from preop | -2.8 | <0.001b) | -2.1 | <0.001b) | ||
| 12 mo | 18 | 1.8±1.2 | 0.300a) | 32 | 2.2±1.8 | |
| Improvement from preop | -2.7 | <0.001b) | -1.9 | <0.001b) | ||
| 24 mo | 12 | 1.8±1.4 | 0.358a) | 20 | 2.4±1.7 | |
| Improvement from preop | -2.3 | <0.001b) | -2.0 | <0.001b) | ||
Table 5.
Back pain (Visual Analog Scale back)
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 5.3±2.6 | 48 | 5.3±2.7 | ||
| 6 wk | 27 | 3.0±2.4 | 0.214 | 34 | 2.3±2.2 | |
| Improvement from preop | -2.6 | <0.001a) | -2.9 | <0.001a) | ||
| 3 mo | 21 | 1.6±1.6 | 0.436 | 37 | 2.0±2.3 | |
| Improvement from preop | -3.3 | <0.001a) | -3.0 | <0.001a) | ||
| 6 mo | 23 | 1.8±2.1 | 0.259 | 39 | 2.4±2.2 | |
| Improvement from preop | -3.3 | <0.001a) | -2.8 | <0.001a) | ||
| 12 mo | 18 | 1.8±2.4 | 0.603 | 32 | 2.2±2.1 | |
| Improvement from preop | -3.2 | <0.001a) | -3.1 | <0.001a) | ||
| 24 mo | 12 | 1.2±2.2 | 0.067 | 21 | 2.8±2.7 | |
| Improvement from preop | -2.9 | <0.001a) | -2.2 | <0.001a) | ||
Table 6.
Oswestry Low Back Pain Disability scores
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 60.8±16.3 | 48 | 62.8±14.1 | ||
| 6 wk | 27 | 48.6±20.5 | 0.638a) | 34 | 50.9±16.3 | |
| Improvement from preop | -13.5 | <0.001b) | -13.1 | <0.001b) | ||
| 3 mos | 20 | 37.4±15.6 | 0.128a) | 38 | 44.1±15.7 | |
| Improvement from preop | -22.3 | <0.001b) | -17.0 | <0.001b) | ||
| 6 mos | 22 | 36.0±12.6 | 0.174a) | 39 | 41.4±17.7 | |
| Improvement from preop | -22.2 | <0.001b) | -21.7 | <0.001b) | ||
| 12 mos | 19 | 32.7±11.9 | 0.085a) | 32 | 40.1±17.8 | |
| Improvement from preop | -25.9 | <0.001b) | -22.1 | <0.001b) | ||
| 24 mos | 12 | 33.0±16.3 | 0.125a) | 21 | 42.4±16.3 | |
| Improvement from preop | -20 | <0.001b) | -21.0 | <0.001b) | ||
Table 7.
Short-Form 36 physical component scores
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 35.8±15.8 | 48 | 36.6±15.3 | ||
| 6 wk | 27 | 47.4±20.4 | 0.833a) | 34 | 46.4±14.7 | |
| Improvement from preop | 11.4 | <0.001b) | 9.2 | <0.001b) | ||
| 3 mo | 21 | 62.7±20.9 | 0.497a) | 36 | 58.7±20.7 | |
| Improvement from preop | 25.1 | <0.001b) | 22.1 | <0.001b) | ||
| 6 mo | 23 | 71.7±17.9 | 0.033a) | 39 | 60.3±22.9 | |
| Improvement from preop | 34.2 | <0.001b) | 24.4 | <0.001b) | ||
| 12 mo | 18 | 74.0±21.4 | 0.202a) | 32 | 65.5±23.0 | |
| Improvement from preop | 38.1 | <0.001b) | 28.1 | <0.001b) | ||
| 24 mo | 12 | 73.0±23.8 | 0.200a) | 22 | 61.4±25.2 | |
| Improvement from preop | 30.7 | <0.001b) | 23.4 | <0.001b) | ||
Table 8.
Short-Form 36 mental component scores
| Period |
Bone morphogenetic protein group (n=29) |
Control group (n=48) |
||||
|---|---|---|---|---|---|---|
| No. of patients | Mean±SD | p-value | No. of patients | Mean±SD | p-value | |
| Preop | 29 | 58.9±18.7 | 48 | 56.7±18.1 | ||
| 6 wk | 26 | 64.0±19.4 | 0.851a) | 34 | 65.0±18.8 | |
| Improvement from preop | 5.8 | 0.007b) | 7.6 | 0.003b) | ||
| 3 mo | 21 | 75.6±15.1 | 0.156a) | 38 | 69.0±20.1 | |
| Improvement from preop | 13.0 | <0.001b) | 10.9 | <0.001b) | ||
| 6 mo | 23 | 74.9±17.2 | 0.215a) | 37 | 68.7±20.7 | |
| Improvement from preop | 16.0 | <0.001b) | 13.0 | <0.001b) | ||
| 12 mo | 17 | 79.7±16.1 | 0.126a) | 32 | 71.3±21.0 | |
| Improvement from preop | 19.9 | <0.001b) | 14.7 | <0.001b) | ||
| 24 mo | 11 | 74.2±22.7 | 0.808a) | 21 | 72.2±17.8 | |
| Improvement from preop | 10.6 | <0.001b) | 20.0 | <0.001b) | ||
References
1. Burkus JK, Transfeldt EE, Kitchel SH, Watkins RG, Balderston RA. Clinical and radiographic outcomes of anterior lumbar interbody fusion using recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976) 2002 27:2396–408.


2. Cahill KS, Chi JH, Day A, Claus EB. Prevalence, complications, and hospital charges associated with use of bone-morphogenetic proteins in spinal fusion procedures. JAMA 2009 302:58–66.


3. Owens K, Glassman SD, Howard JM, Djurasovic M, Witten JL, Carreon LY. Perioperative complications with rhBMP-2 in transforaminal lumbar interbody fusion. Eur Spine J 2011 20:612–7.


4. Mummaneni PV, Pan J, Haid RW, Rodts GE. Contribution of recombinant human bone morphogenetic protein-2 to the rapid creation of interbody fusion when used in transforaminal lumbar interbody fusion: a preliminary report: invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine 2004 1:19–23.


5. Molinari RW, Gerlinger T. Functional outcomes of instrumented posterior lumbar interbody fusion in active-duty US servicemen: a comparison with nonoperative management. Spine J 2001 1:215–24.


6. Okuyama K, Abe E, Suzuki T, Tamura Y, Chiba M, Sato K. Posterior lumbar interbody fusion: a retrospective study of complications after facet joint excision and pedicle screw fixation in 148 cases. Acta Orthop Scand 1999 70:329–34.


7. Fritzell P, Hagg O, Nordwall A, Swedish Lumbar Spine Study Group. Complications in lumbar fusion surgery for chronic low back pain: comparison of three surgical techniques used in a prospective randomized study: a report from the Swedish Lumbar Spine Study Group. Eur Spine J 2003 12:178–89.



8. Villavicencio AT, Burneikiene S, Nelson EL, Bulsara KR, Favors M, Thramann J. Safety of transforaminal lumbar interbody fusion and intervertebral recombinant human bone morphogenetic protein-2. J Neurosurg Spine 2005 3:436–43.



9. Boden SD, Zdeblick TA, Sandhu HS, Heim SE. The use of rhBMP-2 in interbody fusion cages: definitive evidence of osteoinduction in humans: a preliminary report. Spine (Phila Pa 1976) 2000 25:376–81.


10. Haid RW Jr, Branch CL Jr, Alexander JT, Burkus JK. Posterior lumbar interbody fusion using recombinant human bone morphogenetic protein type 2 with cylindrical interbody cages. Spine J 2004 4:527–38.


11. Joseph V, Rampersaud YR. Heterotopic bone formation with the use of rhBMP2 in posterior minimal access interbody fusion: a CT analysis. Spine (Phila Pa 1976) 2007 32:2885–90.


12. Rihn JA, Makda J, Hong J, et al. The use of RhBMP-2 in single-level transforaminal lumbar interbody fusion: a clinical and radiographic analysis. Eur Spine J 2009 18:1629–36.



13. Rodgers SD, Marascalchi BJ, Grobelny BT, Smith ML, Samadani U. Revision surgery after interbody fusion with rhBMP-2: a cautionary tale for spine surgeons. J Neurosurg Spine 2013 18:582–7.


14. Muchow RD, Hsu WK, Anderson PA. Histopathologic inflammatory response induced by recombinant bone morphogenetic protein-2 causing radiculopathy after transforaminal lumbar interbody fusion. Spine J 2010 10:e1–6.

15. Chen NF, Smith ZA, Stiner E, Armin S, Sheikh H, Khoo LT. Symptomatic ectopic bone formation after off-label use of recombinant human bone morphogenetic protein-2 in transforaminal lumbar interbody fusion. J Neurosurg Spine 2010 12:40–6.


16. Wong DA, Kumar A, Jatana S, Ghiselli G, Wong K. Neurologic impairment from ectopic bone in the lumbar canal: a potential complication of off-label PLIF/TLIF use of bone morphogenetic protein-2 (BMP-2). Spine J 2008 8:1011–8.


17. Ahn J, Tabaraee E, Singh K. BMP-2-induced neuroforaminal bone growth in the setting of a minimally invasive transforaminal lumbar interbody fusion. J Spinal Disord Tech 2015 28:186–8.


18. Singh K, Smucker JD, Gill S, Boden SD. Use of recombinant human bone morphogenetic protein-2 as an adjunct in posterolateral lumbar spine fusion: a prospective CT-scan analysis at one and two years. J Spinal Disord Tech 2006 19:416–23.


19. Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone?: an integrated analysis of clinical trials using the LT-CAGE lumbar tapered fusion device. J Spinal Disord Tech 2003 16:113–22.


20. Dimar JR, Glassman SD, Burkus KJ, Carreon LY. Clinical outcomes and fusion success at 2 years of single-level instrumented posterolateral fusions with recombinant human bone morphogenetic protein-2/compression resistant matrix versus iliac crest bone graft. Spine (Phila Pa 1976) 2006 31:2534–9.


21. Potter BK, Freedman BA, Verwiebe EG, Hall JM, Polly DW Jr, Kuklo TR. Transforaminal lumbar interbody fusion: clinical and radiographic results and complications in 100 consecutive patients. J Spinal Disord Tech 2005 18:337–46.


22. Rihn JA, Patel R, Makda J, et al. Complications associated with single-level transforaminal lumbar interbody fusion. Spine J 2009 9:623–9.


23. Patel VV, Zhao L, Wong P, et al. An in vitro and in vivo analysis of fibrin glue use to control bone morphogenetic protein diffusion and bone morphogenetic protein-stimulated bone growth. Spine J 2006 6:397–403.



24. Singh K, Nandyala SV, Marquez-Lara A, et al. Clinical sequelae after rhBMP-2 use in a minimally invasive transforaminal lumbar interbody fusion. Spine J 2013 13:1118–25.


25. Vaidya R, Sethi A, Bartol S, Jacobson M, Coe C, Craig JG. Complications in the use of rhBMP-2 in PEEK cages for interbody spinal fusions. J Spinal Disord Tech 2008 21:557–62.


26. McClellan JW, Mulconrey DS, Forbes RJ, Fullmer N. Vertebral bone resorption after transforaminal lumbar interbody fusion with bone morphogenetic protein (rhBMP-2). J Spinal Disord Tech 2006 19:483–6.


27. Lewandrowski KU, Nanson C, Calderon R. Vertebral osteolysis after posterior interbody lumbar fusion with recombinant human bone morphogenetic protein 2: a report of five cases. Spine J 2007 7:609–14.


28. Toth JM, Boden SD, Burkus JK, Badura JM, Peckham SM, McKay WF. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine (Phila Pa 1976) 2009 34:539–50.


- TOOLS






