Multifidus Muscles Lipid Content Is Associated with Intervertebral Disc Degeneration: A Quantitative Magnetic Resonance Imaging Study
Article information
Abstract
Study Design
Cross-sectional study.
Purpose
To determine the association between fatty degeneration of the multifidus muscle (Mm) and intervertebral disc degeneration (IVDD) using quantitative magnetic resonance imaging (MRI).
Overview of Literature
Few studies have reported on quantitative MRI analysis of the relation between the Mm and IVDD.
Methods
The subjects with chronic low back pain comprised 45 patients (19 males, 26 females; mean age, 63.8±2.0 years; range, 41–79 years). We analyzed the intramyocellular lipids (IMCL) and extramyocellular lipids (EMCL) of the Mm using magnetic resonance spectroscopy. The T2 values of the anterior annulus fibrosus (AF), nucleus pulposus (NP), and posterior AF were evaluated using MRI T2 mapping. We compared the possible correlations of IMCL and EMCL of the Mm with the T2 values of anterior AF, NP, and posterior AF.
Results
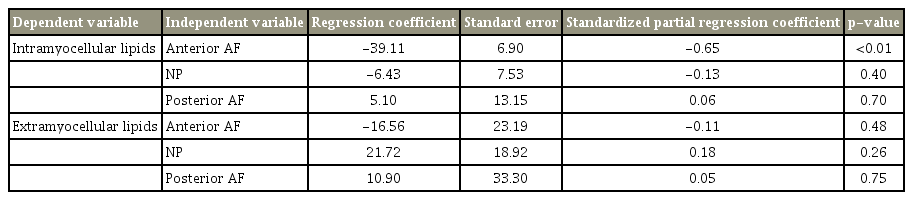
There was a significant negative correlation between IMCL and T2 values of the anterior AF (r=−0.65, p<0.01). There were no significant correlations between the IMCL and T2 values of NP (r=−0.16, p=0.30) and posterior AF (r=0.07, p=0.62). There were no significant correlations between the EMCL and T2 values of the anterior AF (r=−0.11, p=0.46), NP (r=0.15, p=0.32), and posterior AF (r=0.07, p=0.66). After adjustment for age and sex using multiple linear regression analysis, there was a significant negative correlation between the IMCL and T2 values of anterior AF (standardized partial regression coefficient=−0.65, p<0.01).
Conclusions
The results indicated that IMCL of the Mm might be accompanied with anterior AF degeneration. Therapeutic exercises using IMCL of the Mm as evaluation index might have the potential to identify novel targets for the treatment and prevention of IVDD.
Introduction
Low back pain (LBP) is a commonly observed condition and is one of the most serious physiological concerns worldwide [1]. Trunk muscles are important for normal spinal function and are etiologically significant in LBP [2]. In particular, the multifidus muscle (Mm)—the most medially located back muscle and the largest muscle that spans the lumbosacral junction—maintains the erector posture of the trunk as well as abducts and rotates the trunk [3]. Furthermore, intervertebral disc degeneration (IVDD) is considered the principal tissue responsible for LBP [4].
Recently, several studies have performed quantitative evaluation of the Mm fatty degeneration with magnetic resonance spectroscopy (MRS) [5-10] and IVDD with magnetic resonance imaging (MRI) T2 mapping [11-13]. First, MRS analysis of muscle physiology has facilitated detailed analyses of the muscular fat masses by recording the concentration of intramyocellular lipids (IMCL) and extramyocellular lipids (EMCL) [8-10,14-17]. IMCL cannot be visually detected using conventional MRI because they are stored in the form of spheroid droplets in the cytoplasm of muscle cells in close contact with skeletal mitochondria [9,10] and are directly used as an energy source by the mitochondria. Physical inactivity adversely affects skeletal muscle metabolism, manifesting as poor oxidative capacity [18,19], lower glycogen storage, and decreased capillary density [20]. In contrast, EMCL are defined as more or less compact areas of adipose tissue in the subcutaneous layers or along fasciae and are considered metabolically inactive lipid deposits involved in the reduced functionality associated with obesity and a sedentary lifestyle [8,14,15]. Lost muscle strength is associated with EMCL accumulation, which can interfere with sufficient muscle nutrition [8,15,16]. In our previous study [5-7], IMCL using the MRS analyses in the Mm of patients with chronic low back pain (CLBP) was significantly higher than that in the control subjects. Next, MRI T2 mapping utilizes the T2 values for the quantification of moisture content and the collagen sequence breakdown of intervertebral disc (IVD). In a previous work [12], we used MRI T2 mapping to quantify the extent of IVDD, which showed a decrease in the T2 values with increase in the Pfirrmann classification grade [21]. Visual evaluation of the annulus fibrosus (AF) is difficult with ordinary MRI. However, this strategy using T2 values enables us to quantify the degeneration of the AF.
Although fatty degeneration of the Mm as well as IVDD should be a key structural feature of spinal degeneration, few reports exist regarding the MRI analysis of the relation between the Mm and IVDD. The purpose of this cross-sectional quantitative MRI study was to determine the association between the IMCL and EMCL of the Mm and IVDD.
Materials and Methods
The institutional review board of the Sapporo Medical University approved this study (IRB approval no., 262-1074). All the subjects were provided written and verbal explanations of the study and their consent was obtained before study participation.
1. Participants
The subjects comprised patients (>41 years and <79 years old) who had non-specific CLBP defined as pain, stiffness, and discomfort in the lower back from the 12th rib to the lumbar or the lumbosacral area, wherein the source was difficult to identify, and where the symptoms had persisted despite conservative treatments, such as medication and therapeutic exercise, for >3 months. The exclusion criteria included neoplasm, infection, fracture, or history of lumbar vertebral surgery. LBP was assessed using the Visual Analog Scale (VAS, 0–100) after a washout period of minimum 4 weeks. We identified patients whose VAS scores were >30 mm. The subjects comprised 45 patients (19 males, 26 females; mean age, 63.8±2.0 years; range, 41–79 years).
2. Magnetic resonance spectroscopy
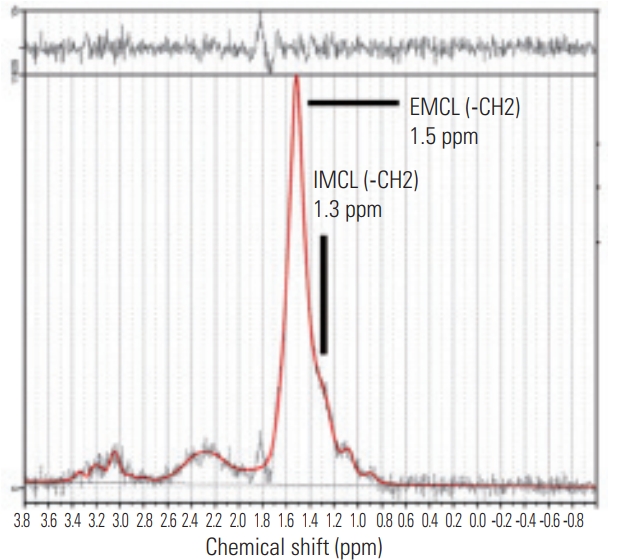
We used the previously described MRI protocol [5-7]. In brief, the Signa HDx 1.5T MRI system (GE Healthcare, Milwaukee, WI, USA) with a spine coil was used to obtain the T2-weighted sagittal and transverse magnetic resonance (MR) images. Using these images, the proton MRS volume of interest (VOI) was positioned in the center of the Mm at L4/5 for the right side (Fig. 1). Single-voxel point-resolved spectroscopy sequence was performed with the following parameters: repetition time (TR), 2,000 ms; echo time (TE), 35 ms; average number of signals, 64; VOI size, 15×15×15 mm (3.4 mL); and acquisition time, 164 seconds. Analyses of MR spectroscopic data were also performed using a previously described method [5-7]. The spectral data obtained were used to measure the IMCL and EMCL using the LCModel software (Stephen Provencher Inc., Oakville, ON, Canada). Data were transferred from the scanners to a Linux workstation, and metabolite quantification was performed with eddy current correction and water scaling. Data for the IMCL (1.3 ppm) and EMCL (1.5 ppm) corresponding to the methylene protons were used for statistical analyses. The assessments of the IMCL and EMCL were automatically scaled to an unsuppressed water peak (4.7 ppm) and expressed in institutional units. These data are graphically displayed with the chemical shift along the x-axis, allowing the identification of the metabolites. Peak intensity has been plotted on the y-axis (Fig. 2). We excluded the subjects with Mm >15% of the %standard deviation of EMCL or IMCL on the LCModel.

Volume of interest for magnetic resonance spectroscopy measurements was set in the multifidus muscle on the right side as indicated on the T2 weighted-image at the L4/5 level.
3. Magnetic resonance imaging T2 mapping
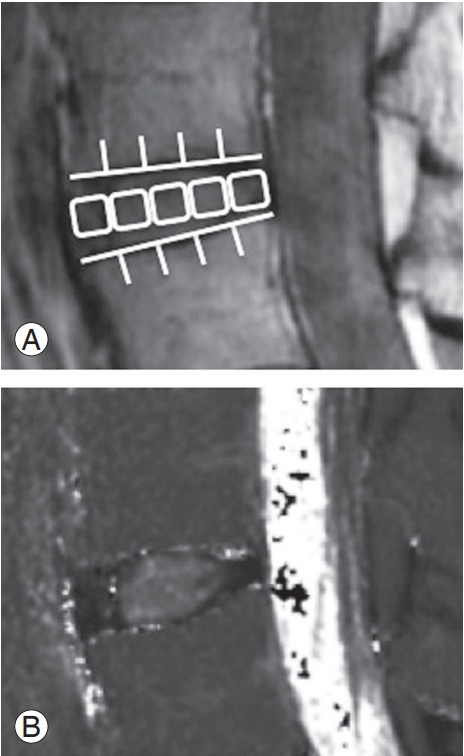
We used the MRI protocol and methods of analysis for MRI T2 mapping that have been previously described [11-13]. Briefly, a T2 map was created at L4/5 level using the T2 values in the midsagittal portion of the sagittal sections centered on the lumbar midline region with optimized 8 echo multi-spin echo (TR/first echo TE, last echo TE, 1,000/14.8, 118.6; receiver bandwidth, ±15.63 kHz; field of view, 22 cm; matrix, 320×256; slice thickness/gap, 4 mm/4 mm; 5 slices; number of excitations, 2; total scan time, 8 minutes and 34 seconds) obtained with an Advantage Workstation (version 4.4, Functool; GE Healthcare). However, the first echo from the multi-spin system was excluded to minimize the effect of the stimulated echo. The T2 map was calculated in each pixel from the signal intensity (SI) in the respective TE using the following calculating formula: SI 1⁄4 e_TE=T2.
For measurement, the disc was divided into five equal areas, designating the front fifth of the anterior AF, the middle fifth of the nucleus pulposus (NP), and the last fifth of the posterior AF, as per previous reports [11-13]. The mean values in the region of interest were measured (Fig. 3). T2 values were measured by a researcher with PhD (H.T. who had 12 years of experience in spine MR image analysis) using MedCalc (ver. 10.2.0.0; MedCalc Software, Mariakerke, Belgium).
4. Statistical analyses
We compared the possible correlations of IMCL and EMCL of the Mm with the T2 values of the anterior AF, NP, and posterior AF using Pearson’s correlation coefficient test. We performed multiple linear regression analysis to eliminate the influence of age and sex. A p<0.05 indicated statistical significance. All the numerical data are expressed as mean±standard error of the mean values.
Results
The mean body mass index was 24.6±0.5 kg/m2. The mean T2 values in IVD were 63.4±2.7 ms for the anterior AF, 73.5±3.3 ms for NP, and 58.0±1.9 ms for the posterior AF. The mean IMCL of the Mm was 10.6±1.62 (×102) mmol/L, and the mean EMCL was 3.72±0.41 (×103) mmol/L. There was a significant negative correlation between the IMCL and T2 values of anterior AF (r=−0.65, p<0.01) (Fig. 4A). There were no significant correlations between IMCL and T2 values of NP (r=−0.16, p=0.30) (Fig. 4B) and posterior AF (r=0.07, p=0.62) (Fig. 4C). There were no significant correlations between the EMCL and T2 values of anterior AF (r=−0.11, p=0.46) (Fig. 5A), NP (r=0.15, p=0.32) (Fig. 5B), and posterior AF (r=0.07, p=0.66) (Fig. 5C). Table 1 shows multiple linear regression analysis adjusted for age and sex. There was a significant negative correlation between the IMCL and T2 values of anterior AF (standardized partial regression coefficient=−0.65, p<0.01).
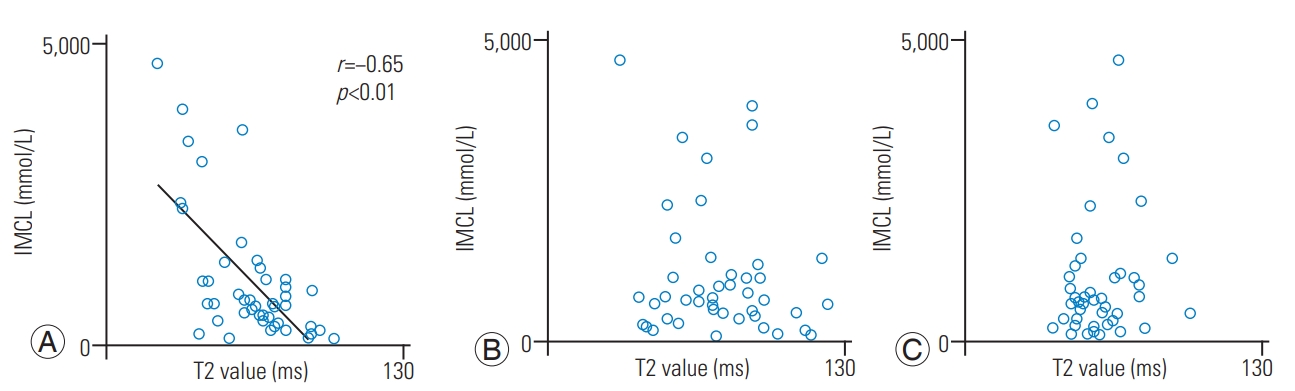
Relationships of the IMCLs and of the multifidus muscle with the T2 values of the anterior AF, NP, and posterior AF. The correlation coefficient between IMCL and anterior AF (A) indicated significant negative correlation. The correlation coefficient between IMCL and T2 values of NP (B) and posterior AF (C) indicated a non-significant correlation. IMCL, intramyocellular lipid; AF, annulus fibrosus; NP, nucleus pulposus.
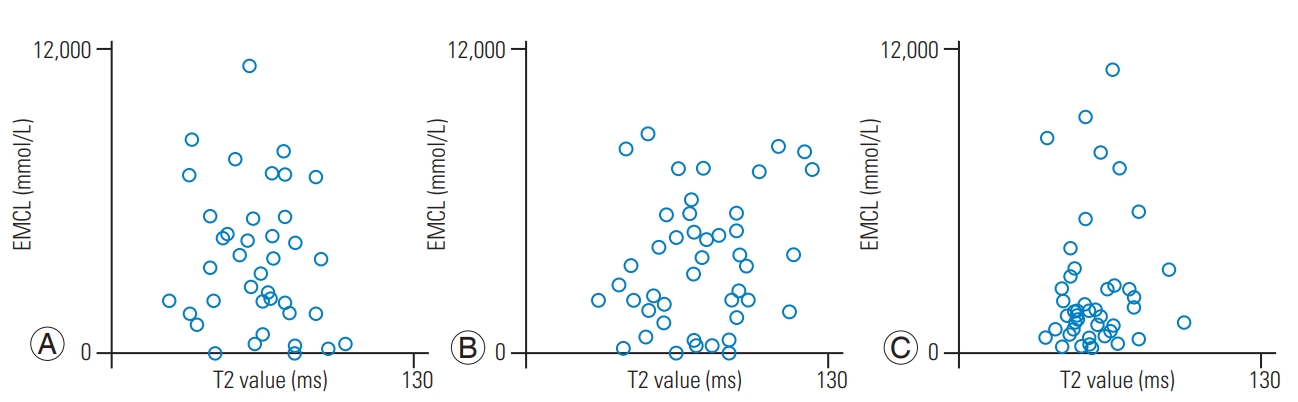
Relationships of EMCLs and of the Mm with the T2 values of anterior AF, NP, and posterior AF. The correlation coefficient between the EMCL and T2 values of anterior AF (A), NP (B), and posterior AF (C) indicated a non-significant correlation. EXCL, extramyocellular lipid; AF, annulus fibrosus; NP, nucleus pulposus.
Discussion
The Mm and IVD are important for the proper functioning of the lumbar spinal column and degeneration in either impairs function [22,23]. Few MRI studies have examined the association between paraspinal muscle fatty degeneration and IVDD. Kader et al. [22] showed a tendency of Mm fatty degeneration being associated with the number of degenerated discs. Teichtahl et al. [23] revealed that severe disc degeneration at all intervertebral levels was associated with high fat content of the paraspinal muscles. Sun et al. [24] reported that Mm atrophy and IVDD were positively correlated at the L3/L4 disc level. However, these studies used visual evaluation to evaluate muscular fatty degeneration and IVDD.
In this study, we used quantitative MRI evaluation methods, such as MRS and MRI T2 mapping, and demonstrated a correlation between IMCL of the Mm and T2 values of anterior AF. Previously, we have identified the associations of spino-pelvic alignment (lumbar lordosis and sagittal vertical axis) with the IMCL of the Mm [5]. The Mm provides two-thirds of the spinal segmental stability [25] and is important for maintaining spinal alignment [26]. In addition, we evaluated the extent of IVDD and compared this with the T2 values in degenerative spondylolisthesis (DS) and no-spondylolisthesis groups; we found that the T2 values decreased in the anterior AF of IVD in the DS group [13]. It is speculated that dysfunction of the Mm leads to anterior gliding because of reduced resistance to anterior shear forces. More mechanical stress loaded at the anterior part of the IVD might lead to pressure on the anterior AF. Thus, the Mm as well as anterior AF of IVD are important for proper functioning of the lumbar spinal column and are cross-correlated to each other.
IVDD [27] and collapsed vertebral bodies [28] irreversibly develop with advancing age; however, an increase in the IMCL of the Mm can probably improve if conservative treatments, such as therapeutic exercise, are initiated. There is a correlation between IMCL of the Mm and the T2 values of anterior AF; therefore, it is expected that therapeutic exercises might have the potential to identify novel targets for the treatment and prevention of IVDD.
This study has certain limitations. First, this study has a cross-sectional design; thus, it cannot be determined whether associations between IMCL increase of the Mm and IVDD are a cause or the result of one another. Longitudinal studies will help address such concerns. Second, we did not perform any evaluation about other factors involved in IVDD, such as smoking, diabetes mellitus, hypertension, and physical activity.
Conclusions
The results indicated that the IMCL of the Mm might be accompanied with posterior AF degeneration. Therapeutic exercises using IMCL of the Mm as evaluation index might have the potential to identify novel targets for the treatment and prevention of IVDD.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: IO, TT, HT; acquisition of data: IO, HT; analysis and interpretation of data: IO, HT; drafting the article: IO, TT; critically revising the article: all authors; reviewed submitted version of manuscript: all authors; approved the final version of the manuscript on behalf of all authors: TT; statistical analysis: IO; administrative/technical/material support: TM, TO, YT, MY, TY; and study supervision: TY.