Prevention of Adjacent Segmental Disease after Fusion in Degenerative Spinal Disorder: Correlation between Segmental Lumbar Lordosis Ratio and Pelvic Incidence–Lumbar Lordosis Mismatch for a Minimum 5-Year Follow-up
Article information
Abstract
Study Design
Retrospective study.
Purpose
Associations among risk factors related to adjacent segmental disease (ASD) remain unclear. We evaluated the risk factors and segmental lordosis ratio to prevent ASD developing after lumbar spinal fusion.
Overview of Literature
Risk factors related to ASD development are age, sex, obesity, pre-existing degeneration, number of fusion segments, and decreased postoperative lumbar lordosis (LL). However, the associations among these factors are still unclear and should be clearly identified.
Methods
We retrospectively reviewed data on 274 patients who underwent lumbar spinal fusion of three segments or below for lumbar degenerative disease from January 2010 to December 2012, with over 5 years of follow-up. Patients with preoperative sagittal vertical axis (SVA) >5 cm were excluded due to sagittal imbalance. A total of 37 patients with ASD and 40 control patients (CTRL) were randomly selected in a similar distribution of matching variables: age, sex, and preoperative degenerative changes. Sex, age, number of fusion segments, radiologic measurements, L4–5–S1/L1–S1 LL ratio, and spinopelvic parameters (pelvic incidence [PI], pelvic tilt [PT], sacral slope [SS], and SVA) were analyzed. Logistic regression was used to analyze the correlation between PI–LL mismatch and L4–5–S1 segmental lordosis rate.
Results
No significant difference was found between ASDs and CTRL groups regarding age, sex, number of fusion segments, fusion method, and preoperative and postoperative spinopelvic parameters (PI, SS, PT, and LL). However, regarding the L4–5–S1/L1–S1 lordosis ratio, 50% (p=0.045), 60% (p=0.031), 70% (p=0.042), 80% (p=0.023), and 90% (p=0.023) were statistically significant; <20% (p=0.478), 30% (p=0.223), and 40% (p=0.089) were not statistically significant. In the postoperative PI–LL <10 group, ASD occurred less frequently than in the PI–LL >10 group, and the difference was statistically significant (p=0.048).
Conclusions
Patients with a postoperative L4–5–S1/L1–S1 lordosis ratio >50% had less occurrence of ASD. Correcting LL according to PI and physiologic segmental lordosis ratio is important in preventing ASD.
Introduction
Spinal fusion has become a common treatment for numerous pathologic conditions of the human spine [1-4]. However, spinal fusions may increase the stress on the nonoperated adjacent lumbar segments, causing a common complication of adjacent segmental degeneration in long-term follow-up [5-7].
Cheh et al. [8] found that 43% of patients showed radiographic adjacent segmental degeneration and 24% had symptomatic adjacent segmental disease (ASD), whereas 6.3% had clinical signs without radiographic evidence. The reported ASD rates in the literature range from 2.6% to 30.3% [2,9]. We used the term ASD for symptomatic adjacent segmental degeneration. The development of ASD is problematic because it can necessitate further surgical intervention and adversely affect functional outcomes [9]. For this reason, there has recently been a growing interest in the risk factors to prevent and treat degenerative changes in adjacent segments after spinal fusion. Known risk factors related to the development of ASD were age, sex, obesity, pre-existing degeneration, number of fusion segments, and decreased postoperative lumbar lordosis (LL) [2,4,10,11]. However, the associations among these factors remain unclear and should be clearly identified.
The importance of spinopelvic balance and its implications on the clinical treatment of patients with lower back pain have been shown in recent studies [12-15]. However, little is known about how spinopelvic alignment affects adjacent segmental stress or how this may contribute to lumbar disk degeneration. Since the relationship between pelvic incidence (PI) as a morphologic parameter and LL appears important for the sagittal profile of the spine, it may also account for different loading patterns in the lumbar spine, which may be relevant for the development of adjacent segmental degeneration and disease. Rothenfluh et al. [16] demonstrated that patients with ΔPI–LL >10 have a 10 times higher risk for ASD development, while a PI–LL mismatch of ≥10 indicated that patients are likely to have either imbalance or compensating.
Furthermore, in terms of regional balance, Bernhardt and Bridwell [17] showed that >60% of LL is created by the disks at L4–5 and L5–S1 in the normal spine, which contribute −20° and −28° to the regional lordotic measurement. The normal range of the total lumbar lordotic angle according to Jackson and McManus [18] is 33°–88° (L1–S1) and 33°–79° (L1–S1) according to Peterson et al. [19] Kim et al. [20] analyzed the normal mean value of LL in the Asian spine, which was 47°, ranging from 23°–65°. However, LL has a large standard deviation and different normal values for each individual; hence, it is inappropriate to compare. We used ratio to discuss regional lordosis. We focused on correcting L4–5–S1 segmental lordosis based on the above finding to significantly reduce the incidence of ASD.
To the best of our knowledge, no study has demonstrated the correlation between PI–LL mismatch and segmental lordosis ratio (L4–5–S1/L1–S1 lordosis ratio) for the prevention of ASD.
Materials and Methods
We hypothesized that PI–LL <10 and correction of L4–5–S1 segmental lordosis ratio to physiologic lordosis may prevent the incidence of ASD. This study was retrospective in nature and we obtained final approval of exemption from Eulji University Hospital institutional review board. and the written informed consets were obtained.
A total of 274 adults with lumbar spinal degeneration surgically treated at a single institution between January 2010 and December 2012 were studied. All patients were followed for a minimum of 5 years. Inclusion criteria were patients who underwent primary lumbar fusion of three segments or lower between L2 and S1 for lumbar degenerative disease and underwent revision surgery for symptomatic ASD during follow-up. All study patients underwent standing whole-spine lateral X-ray with inclusion of the femoral heads. Indications for surgery were degenerative lumbar disorders with leg pain and claudication. Our exclusion criteria were patients who underwent previous surgery and had been followed for <5 years postoperatively patients with a preoperative sagittal vertical axis (SVA) >5 cm were excluded because of sagittal imbalance. In addition, we excluded patients with degenerative lumbar scoliosis more severe than 20°.
Sagittal imbalance was measured by the standing whole-spine lateral X-ray taken according to the method recommended by the preoperative spinal deformity study group. Magnetic resonance imaging (MRI) was performed in patients with back pain and neurogenic claudication and showed improvement in symptoms for a certain period of time after initial surgery to exclude cases of degenerative changes in adjacent segments without clinical symptoms. ASD was diagnosed when spinal canal stenosis and disk herniation were observed at the adjacent segment on MRI. The patient underwent revision surgery for at least 3 months in which symptoms persisted.
Of 274 patients, 40 control patients (CTRL) were randomly selected from the patient pool of lumbar spinal fusion procedures that met the above inclusion criteria, except for revision surgery, to be similar in the distribution of age, sex, and preoperative degenerative changes to those of the ASDs group with the same exclusion criteria. Patients were included in the CTRL group only if they had a minimum follow-up of 5 years, no signs of symptomatic ASD at last follow-up, similar distribution of levels and number of segments fused, and a comparable degree of disk degeneration in the prospective adjacent segment preoperatively as assessed on MRI.
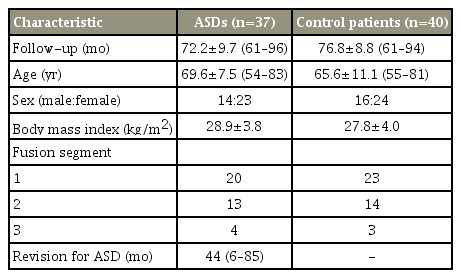
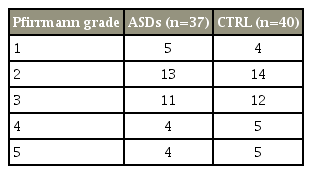
In total, 77 patients (37 ASDs, 40 CTRL) were included who demonstrated adequate follow-up and radiographs with preoperative MRI of the lumbar spine. All patients who underwent posterolateral instrumented fusion with pedicle screws showed union during follow-up. Details on the patients’ demographic data are given in Table 1. The Pfirrmann grade was measured to prove that there was no difference in the degree of preoperative disk degeneration between ASDs and CTRLs and the results demonstrated there was no significant difference between the two groups (Table 2). Similar groups in the distribution of their matching variables were selected before measurements were performed on radiographs to minimize the selection bias.
Sex, age, number of fusion segments, and radiological measurements, including segmental lordosis ratio and pelvic parameters, were analyzed. The spinopelvic alignment was characterized by LL, PI, sacral slope (SS), and pelvic tilt (PT) values. The differences between PI and LL were computed, and for the grouping of patients, a PI–LL threshold of 10 was adopted based on previous studies.
LL was measured from L1–S1 and L4–S1 for the assessment of L4–5–S1/L1–S1 segmental lordosis ratio (Fig. 1). In addition, according to the number of fusion segments after the first operation, when fusion was performed from the L3 level, the lordotic angle was measured between L3 upper end plate and S1; when the fusion was performed from the L4 level, the lordotic angle was measured between L4 upper end plate and S1. The results were compared by >30%, 40%, 50%, 60%, and 70% (Table 3).
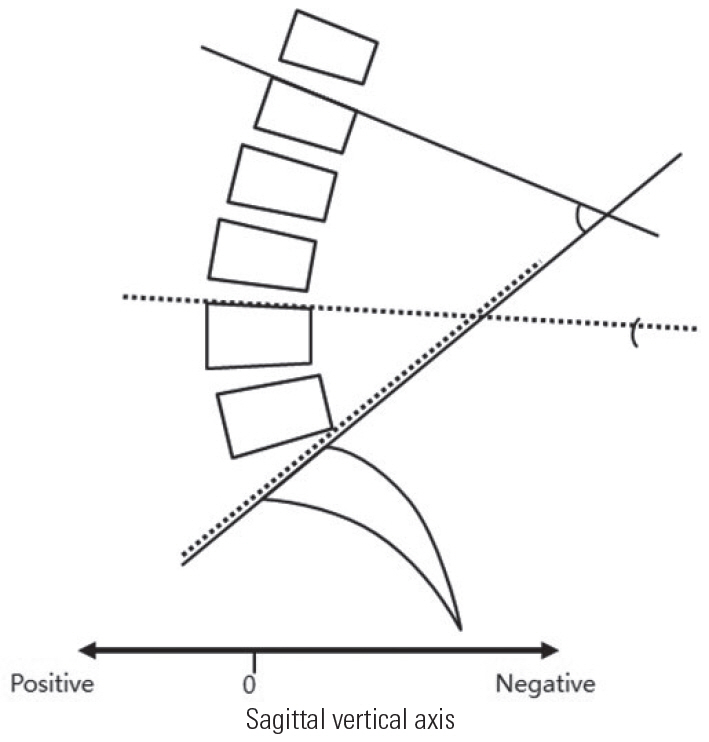
Segmental lordosis ratio was performed as shown. The solid line indicates L1–S1 lumbar lordosis, the dotted line indicates L4–S1 lumbar lordosis.
Radiographic measurements were performed using m-view (Infinity Healthcare Co., Ocean City, NJ, USA) on the Hospital’s Picture Archiving and Communication System. Measurements were obtained twice for each author and the mean values were calculated. Reliability was classified as small (0–0.24), low (0.25–0.49), medium (0.50–0.69), excellent (0.70–0.89), and best (0.90–1.0) depending on the coincidence coefficient in the group. The interobserver reliability of this study was estimated to be 0.93.
Statistical analysis was performed using PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) for the two groups. The Mann-Whitney U-test and the two-sample Kolmogorov-Smirnov test were used to confirm the statistical significance of the measurements in both groups. We used logistic regression analysis to analyze the correlation between PI–LL mismatch and L4–5–S1 segmental lordosis rate. The significance level was p<0.05
Results
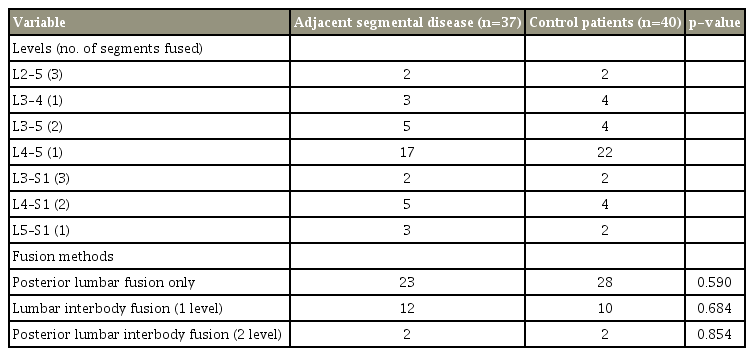
No statistically significant difference was found between the ASD and CTRL groups on age, sex, body mass index, and number of fusion segments (Tables 1, 2). Among the fusion methods, there were 23 cases of posterolateral fusion, 12 one-level posterior lumbar interbody fusion (PLIF), and two two-level PLIF in the ASD group, and 28, 10, and two, respectively, in the CTRL group. Moreover, no statistically significant difference was found between the two groups (Table 4).
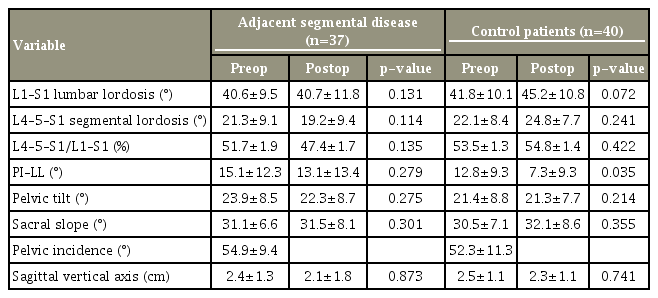
On our radiologic measurements, the comparison of pre- and postoperative values showed no significant differences in terms of spine parameter between the ASD and CTRL groups for L1–S1 LL and L4–5–S1 LL. No significant differences were found between the two groups regarding pelvic or postoperative parameters. However, PI–LL in the CTRL group showed a statistically significant difference between preoperative and postoperative values (Table 5).
We analyzed the correlation between L4–5–S1 segmental lordosis ratio and ASD. The results were >30% (p=0.223), 40% (p=0.089), 50% (p=0.045), 60% (p=0.031), 70% (p=0.042). When the ratio was >50% (p=0.045), a statistical significance was observed and ASD occurred less frequently when the L4–5–S1 segmental lordosis ratio was >50% (Table 3, Figs. 2–5).

(A) Preoperative radiograph; (B) 2 weeks postoperatively; and (C) final follow-up 4 years 8 months postoperatively. A 56-year-old woman with one segment fusion. Preoperative measurements were PI–LL -6, segmental lordotic ratio 74%. Postoperative measurements were PI–LL -2, segmental lordotic ratio 62%. Patient with PI–LL <10 and good segmental lordotic ratio showed a good prognosis without adjacent segmental disease. PI, pelvic incidence; LL, lumbar lordosis.
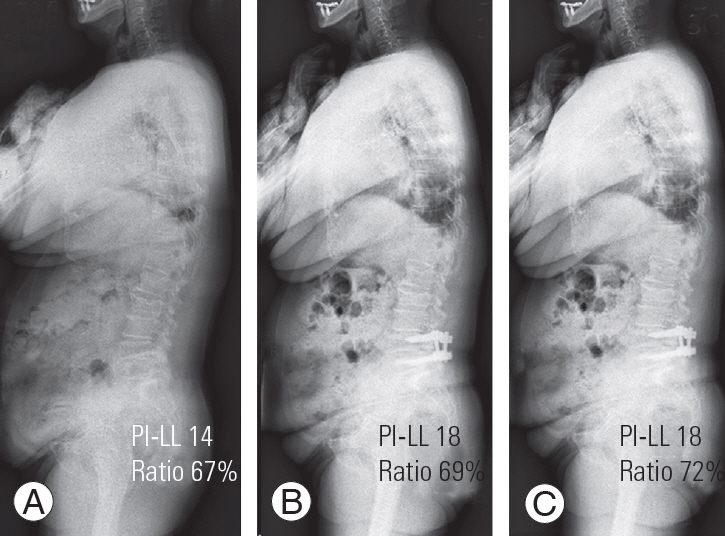
(A) Preoperative radiograph; (B) 2 years 3 months postoperatively; and (C) postoperative radiograph of revision surgery. A 72-year-old woman with one segment fusion. Preoperative measurements were PI–LL 14, segmental lordotic ratio 67%. Postoperative measurements were PI–LL 18, segmental lordotic ratio 69%. Patient with PI–LL >10, but good segmental lordotic ratio showed good prognosis and underwent revision surgery. PI, pelvic incidence; LL, lumbar lordosis.

(A) Preoperative radiograph. (B) 2 years 3 months postoperatively. (C) Postoperative radiograph of revision surgery. A 63-year-old woman with 1 segment fusion and posterior lumbar interbody fusion. Preoperative measurements were PI–LL 5, segmental lordotic ratio 36%. Postoperative measurements were PI–LL 8, segmental lordotic ratio 21%. Patient with PI–LL <10, but poor segmental lordotic ratio showed poor prognosis and underwent revision surgery. PI, pelvic incidence; LL, lumbar lordosis.

(A) Preoperative radiograph; (B) 6 months postoperatively; and (C) postoperative radiograph of revision surgery. A 75-year-old woman with one segment fusion. Preoperative measurement were PI–LL 20, segmental lordotic ratio 25%. Postoperative measurements were PI–LL 15, segmental lordotic ratio 21%. Patient with PI–LL >10 and poor segmental lordotic ratio showed poor prognosis and underwent revision surgery. The patient had an ASD at 6 months postoperatively and underwent revision at the early stage. This case suggested that if the PI–LL and the regional lordosis were not aligned well, the ASD could occur more rapidly. PI, pelvic incidence; LL, lumbar lordosis; ASD, adjacent segmental disease.
PI–LL was <10 in 12 and 24 patients and >10 in 25 and 16 in the ASD (n=37) and CTRL (n=40) groups, respectively. Of the 77 patients, the rate of ASD occurrence was 33.3% (12/36) in the postoperative PI–LL <10 group and 61% (25/41) in the PI–LL >10 group. In the postoperative PI–LL <10 group, ASD occurred less than in the PI–LL>10 group, and this was statistically significant (p=0.048) (Table 3).
In the PI–LL <10 group, if the segmental lordosis ratio was <50%, ASD occurred frequently (ASD group, 75% [9/12]; CTRL group, 37.5% [9/24]). However, in the PI–LL >10 group, if the lumbar segmental lordosis ratio was >50%, ASD occurred less frequently (ASD group, 24% [6/25]; CTRL group, 62.5% [10/16]). We found that the incidence of ASD was significantly reduced in patients with a PI–LL <10 and a L4–5–S1/L1–S1 segmental lordosis ratio of >50% (Table 3).
Discussion
Hilibrand and Robbins [5] described ASD as a radiologic change in the adjacent segment only and a clinical symptom accompanied by radiologic change. Recently, many studies were done on the risk factors related to ASD after fusion [1-4,10,11]. The development of ASD is problematic and adjacent segmental degeneration and disease have a significant clinical impact; hence, numerous studies have aimed at identifying the risk factors [9].
The risk factors for ASD development include patient factors, such as age, sex, and bone mineral density; preoperative factors, such as instability and intervertebral disc herniation; and postoperative factors, such as sagittal alignment, number of fusion segments, and fusion method [1-4,10,11]. However, the risk factors with relatively clear implications on subsequent surgical management have not been identified.
Many researchers thought that there would be more changes in the adjacent segments with increasing age. Lee et al. [2] reported that the incidence of ASD was higher in patients >60 years old, and Aota et al. [21] found that the incidence of ASD was higher in patients >55 years old. However, controversies exist regarding the relationship between the occurrence of segmental diseases and age [4]. In our study, no statistically significant difference was noted (p=0.424) between the mean age of patients with (72.2±9.7 years) and without (76.8±8.8 years) ASD. Although older ages are likely to influence the development of ASD, considering that both groups are >60 years old, it would be unreasonable to evaluate that older age affects ASD in this study.
Kim et al. [22] reported no significant differences between the posterior interbody fusion and posterolateral fusion in the incidence of ASD. However, Lee et al. [3] and Brodsky et al. [23] compared posterior interbody fusion and posterolateral fusion, and the incidence of ASD was lower in posterolateral fusion. In our study, no significant difference was noted between posterior interbody fusion and posterolateral fusion in the incidence of ASD (Table 4).
Furthermore, no statistically significant difference was found between the ASD (1.48±0.34) and CTRL groups (1.40±0.27) in terms of fusion segment number (p=0.486). Moreover, no correlation between fusion segment number and occurrence of ASD was found in patients with threesegment fusion or less.
In addition, there is a correlation that the incidence of ASDs is increased because stress is highly concentrated on the adjacent segments as multiple segments are fused. Kim et al. [1] and Rohlmann et al. [24] reported that the cause of increased compensatory motion of adjacent segments in the long segmental fusion was more influenced by sagittal or coronal imbalance than by physical factors. As the importance of LL correction is emphasized when long segment fusion is needed, performing anterior fusion together is becoming increasingly common.
PI is a unique radiologic index and predictor of the amount of LL required to assume a balanced sagittal posture. It is a very important factor in evaluating sagittal balance [18]. Recently, Schwab et al. [25] reported sagittal modifiers, including SVA, PT, and PI–LL, which are indicators of health-related quality of life. The International Spine Study Group has recently reported the relevance of PI–LL mismatch in adult deformity. A significant difference was found between these indicators and thus their correction is an important factor in surgical treatment. However, studies on altered kinematics and biomechanics after lumbar spinal fusion did not focus on spinopelvic alignment or sagittal imbalance, but instead investigated hypermobility and increased loads in the adjacent segment [16]. The appropriate lumbar lordotic angle is known as PI±9°. Rothenfluh et al. [16] demonstrated that patients with ΔPI–LL >10 have a 10 times higher risk for ASD development and PI–LL mismatch of ≥10 indicated that patients are either likely to have imbalance or compensating. In this study, the relationship between the degree of lumbar lordotic deviation and ASD occurrence was evaluated using PI, and when PI–LL was <10, it was statistically significant (p=0.048). Other spinopelvic parameters, including SS, PT, and SVA, did not show a statistically significant difference because we excluded patients with sagittal imbalance.
In terms of regional lordosis, Bernhardt and Bridwell [17] reported that LL >60% is created by L4–5 and L5–S1 disks. In the present study, when L4–5–S1/L1–S1 segmental lordosis ratio was approximately 50%, ASD occurred less frequently. We believe that LL has a large standard deviation and different normal values for each individual; hence, it is inappropriate to compare and so we used ratio to discuss regional lordosis. Considering functional neutrality, a statistically significant result could be obtained in a wider range than the idea that occurrence of ASD would be less frequent if the ratio was 60%. When the ratio was 50% (p=0.045), a statistical significance could be observed. We believe that is because our study subjects are an elderly population with normal progressive spinal degeneration; thus, 50% appears to be acceptable.
Sagittal alignment is important to prevent the development of ASD, and sagittal plane balance after lumbar fusion is an important factor that affects the motion of adjacent segments and is directly related to LL. In this study, when PI–LL was satisfactorily corrected to <10, but the lumbar segmental lordosis ratio was not, ASD frequently occurred in the corrected group (ASD group, 75% [9/12]; CTRL group, 37.5% [9/24]). We suggest that for reducing the incidence of ASD, lumbar segmental lordosis ratio should be evaluated as well as the appropriate LL angle according to each individual’s PI.
The major limitation of this study was the lack of representation of the population as a whole, due to the small sample size and the fact that the patients were followed for >5 years, which is a long period of time.
L4 was not the only transition point in the lumbar spine [26]. That means that lower lumbar curvature may share less proportion in the whole lumbar spine in some cases. In surgical methods, we used only posterior lumbar fusion and PLIF to restore LL. Further studies were needed on LL restoration through surgical procedures, such as anterior lumbar, direct lateral, and transforaminal lumbar interbody fusion. In addition, it was difficult to determine the factors affecting the resultant value in various aspects. Moreover, evaluations of actual function, satisfaction, and other complications were insufficient. However, given that the evaluation was made only by radiologic measurement, relatively objective data could be obtained.
Conclusions
After a fusion operation for a degenerative spine, patients with a postoperative L4–5–S1/L1–S1 segmental lordosis ratio of >50% experienced less frequent occurrences of ASD. Correcting the LL according to PI and physiologic lordosis ratio is important in preventing ASD.
Notes
No potential conflict of interest relevant to this article was reported.