 |
 |
- Search
| Asian Spine J > Volume 14(4); 2020 > Article |
|
Abstract
Fig. 1.
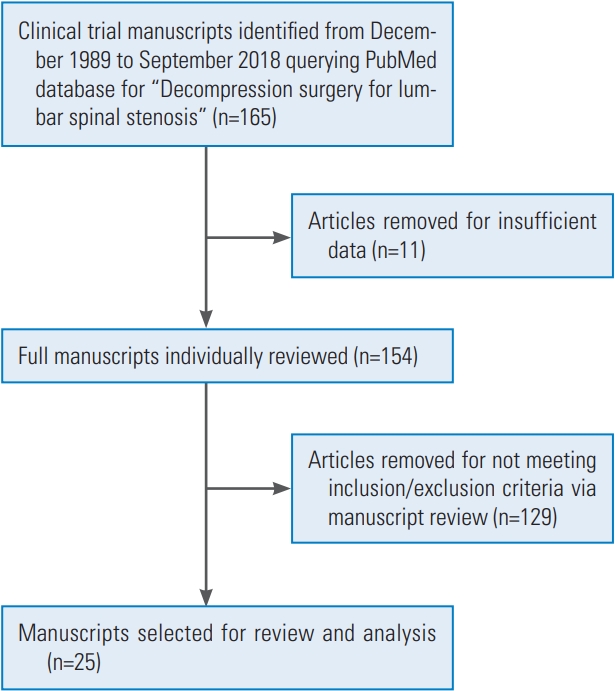
Fig. 2.
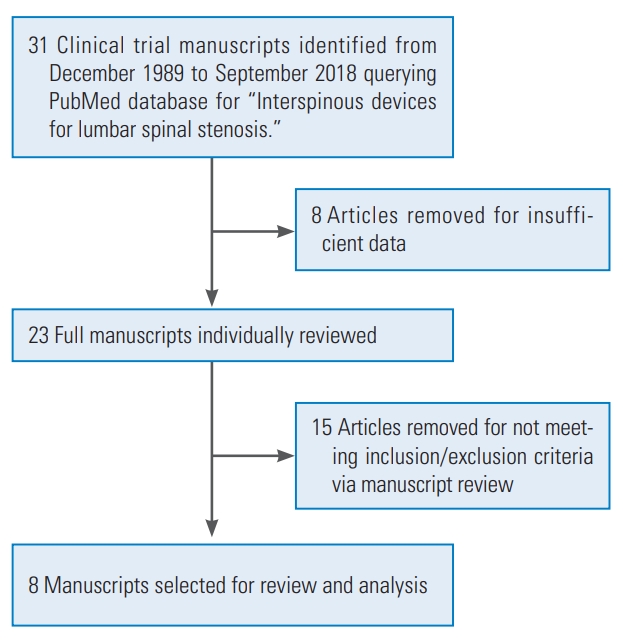
Fig. 3.
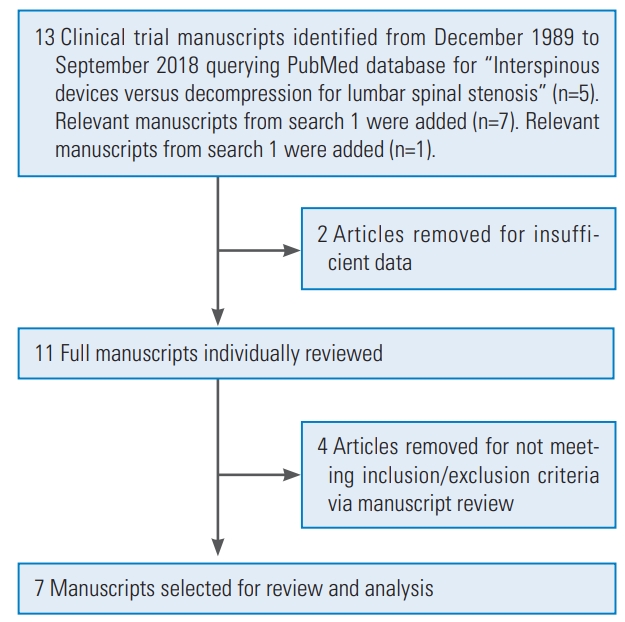
Fig. 4.
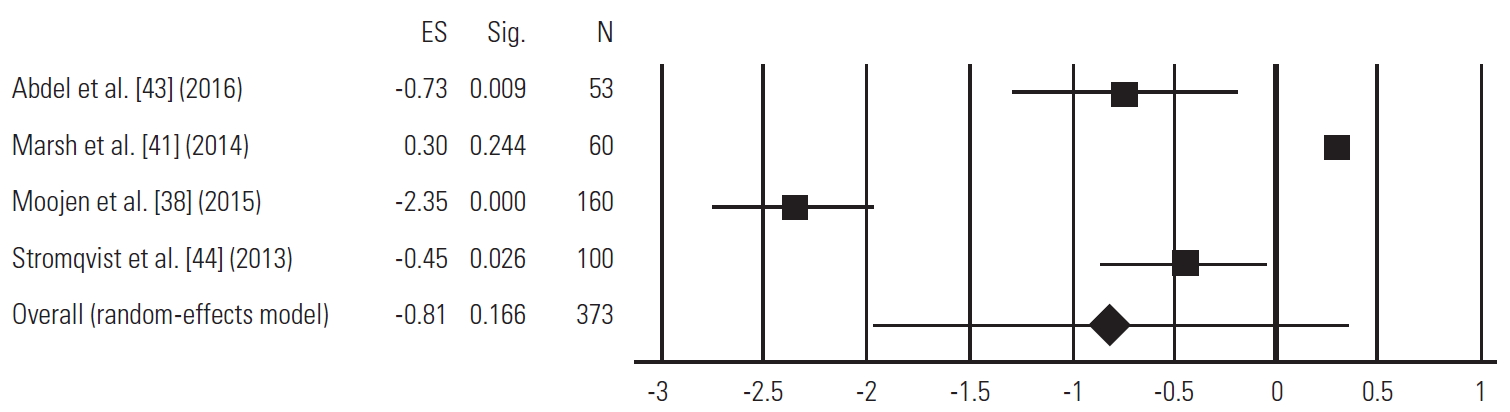
Table 1.
| Author | Year | Type of clinical trial | Type of decompressive surgery | Sample size | Age (yr) | VAS | ODI | JOA | Mortality/complications | FU |
|---|---|---|---|---|---|---|---|---|---|---|
| Minamide et al. [5] | 2018 | PCS | Microendoscopic laminotomy | 242 (101 M, 141 F) | Average, 68.1; range, 46 to 85 | NA | NA | Preop, 14.4±4.3; postop at last FU, 23.3±4.8 | Dural tear (3); transient neuralgia (3); epidural hematoma (4); infection (1) | Average: 4.6 yr |
| Minamide et al. [4] | 2017 | PCS | MID | 122 (57 M, 65 F) | Average, 70.4±8; range, 46 to 88 | Preop, 55.9±28.1; postop at last FU, 35.0±30.1 | NA | Preop, 13.4±4.4; postop at last FU, 20.5±5.3 | NA | Average: 2.4 yr |
| Benyamin et al. [6] | 2016 | RCT | MID | 149 (74 M, 75 F) | Average, 75.6±7.0 | NA | Preop, 53.0±12.9; postop at 1 yr, 37.0 | NA | Non-serious procedural hemorrhage (1) | 6 mo, 1 yr, 2 yr |
| Staats et al. [7] | 2016 | RCT | MID | 149 (74 M, 75 F) | Average, 75.6±7.0 | NA | Preop, 53.0±12.9; postop at 6 mo, 34.5 | NA | Procedural hemorrhage (1); procedural pain (1) | 6 mo |
| Delitto et al. [18] | 2015 | RCT | Traditional decompression with laminectomies, facet resection and neuroforaminotomy | 87 (44 M, 43 F) | Average, 66.6±10.5 | NA | Preop, 42.6; postop at 24 mo, 25.2 | NA | Allergy (1); blood loss (1); respiratory tract infection (3); reoperation of lumbar spine (3); worsening symptoms (2); wound healing/site infection (7) | 24 mo |
| Nerland et al. [8] | 2015 | PCS | MID, open laminectomy | MID: 471 (249 M, 222 F); laminectomy: 414 (209 M, 205 F) | MID: average, 66.6; laminectomy: average, 70.1 | NA | MID: preop, 38.0; postop at 1 yr, 19.6; laminectomy: preop, 42.1; postop at 1 yr, 25.0 | NA | MID: dural tear/spinal fluid leak (11); postop hematoma (1); cardiovascular complications (1); wound infection (8); UTIs (17); micturition problems (3); pneumonia (5); Laminectomy: dural tear/spinal fluid leak (21); postop hematoma (4); anaphylactic reaction (1); wound infection (11); UTIs (20); micturition problems (15); pneumonia (2); pulmonary embolism (1); DVT (2) | 10 wk, 6 mo, 12 mo, 24 mo |
| Komp et al. [9] | 2015 | RCT | MI, FI | Total: 180 (91 M, 69 F); MI: 80; FI: 80 | Total: average, 62; range, 41 to 84 | MI: preop, 25; postop at 24 mo, 12; FI: preop, 23; postop at 24 mo, 17 | MI: preop, 88; postop at 24 mo, 30; FI: preop, 84; postop at 24 mo, 28 | NA | MI: transient dysesthesia (7); transient urinary retention (3); dura injuries (3); increased foot dorsiflexion paresis (2); epidural hematoma (1); delayed wound healing (2); soft tissue infections (2); revision surgery with fusion (2); FI: transient dysesthesia (4); transient urinary retention (1); dura injuries (2); increased foot dorsiflexion paresis (1); delayed wound healing (1); revision surgery (1) | 3 mo, 6 mo, 12 mo, 24 mo |
| Mobbs et al.[16] | 2014 | RCT | ULBD, traditional laminectomy | ULBD: 27; laminectomy: 27 | ULBD: average, 72.7±10.4; laminectomy: average, 65.8±14.3 | NA | ULBD: preop, 51.4±19.4; mean improvement at last FU, 28.6±27.7; laminectomy: preop, 46.6±18.9; mean improvement at last FU, 17.8±15.4 | NA | ULBD: dural tear (1); laminectomy: foot drop (1); hematoma (1); dural tear (1) | ULBD: average: 36.9±4.3 mo; laminectomy: average: 44.3±15.0 mo |
| Hajasek-aran et al. [28] | 2013 | HCT | LSPD, midline decompression | LSPD: 28 (16 M,12F); midline decompression: 23 (14M,9F) | LSPD: average, 57.25±11.23; range: 35 to 76; midline decompression: average, 54.48±8.21; range, 39 to 71 | LSPD: preop, 5.35; postop at last FU, 2.46; midline decompression: preop, 5.43; postop at last FU, 2.96 | NA | LSPD: preop, 6.96; postop at last FU, 10.75; midline decompression: preop, 6.69; postop at last FU, 11.39 | No major complications | Average: 14.2±2.9 mo |
| Liu et al. [19] | 2013 | RCT | M-ULBD, traditional laminectomy | M-ULBD: 27 (15 M, 12 F); laminectomy: 29 (18 M, 11 F) | M-ULBD: average, 59.4±4.7; range, 49 to 71; laminectomy: average, 61.1±3.1; range, 52 to 69 | M-ULBD: preop, 5.6±1.7; postop, 1.0±0.5; laminectomy: preop, 7.9±1.3; postop at last FU, 2.6±0.7 | NA | M-ULBD: preop, 11.6±2.6; postop, 26.7±2.1; laminectomy: preop, 10.9±1.0; postop at last FU, 24.3±2.5 | M-ULBD: dural tear (3); laminectomy: dural tear (1) | Average: 2yr |
| Deer et al, [26] | 2012 | PCS | Mild percutaneous lumbar decompression | 46 (17 M, 29 F) | Average, 66.1; range, 46 to 80 | Preop, 6.9±0.6; postop at 1 yr, 4.0±1.0 | Preop, 49.4±2.5; postop at 1 yr, 32.0±5.8 | NA | No major complications | 12 wk, 6 mo and 1 yr |
| Guilfoyle et al. [20] | 2012 | RCT | Traditional laminectomy w/o epidural fentanyl, traditional laminectomy with epidural fentanyl | Decompression w/o fentanyl: 31 (14 M,17 F); decompression with fentanyl: 29 (16 M,13 F) | Decompression w/o fentanyl: average, 65.7±11.5; decompression with fentanyl: average, 69.9±9.8 | Decompression w/o fentanyl: preop, 4.1±3.0; postop in recovery room, 4.7±2.4; decompression with fentanyl: preop, 3.4±2.9; postop in recovery room, 2.6±2.7 | NA | NA | Decompression w/o fentanyl: itching (2); nausea/vomiting (4); decompression with fentanyl: itching (1); nausea/vomiting (3); urinary catheter (3) | In “recovery”, before postop day 1 |
| Wong et al.[29] | 2012 | Case series | Mild interlaminar decompression | 17 (10 M,7 F) | Average, 73.1; range, 63 to 86 | Preop, 7.6; postop at 1 yr, 2.3 | Preop, 48.4; postop at 1yr, 21.7 | NA | No major complications | 1 yr |
| Komp et al. [45] | 2011 | Case series | Full-endoscopic unilateral decompression | 90 (41 M, 49 F) | Average, 61; range, 43 to 81 | Preop, 23; postop at 24 mo, 17 | Preop, 85; postop at 24 mo, 31 | NA | Dysesthesia (5); urinary retention (2); dural injuries (2); increase in foot dorsiflexion paresis (1); repeat surgery (2) | 3 mo, 6 mo, 12 mo, 24 mo |
| Jakola et al.[21] | 2010 | PCS | Traditional laminectomy | 101 (51 M, 50 F) | Average, 75.3±4.1; range, 70 to 86 | Preop, 55.7; postop at 12 mo, 35.9 | Preop, 44.2; postop at 12 mo, 27.9 | NA | Dural tear (9); superficial wound infection (3); perioperative death (1); UTI (1); deep wound infection (2); myocardial infarction (1); gastric ulcer (3) | 3 mo, 12 mo |
| Chopko et al.[10] | 2010 | PCS | Ultra-minimally invasive lumbar decompression | 75 (29 M, 46 F) | Average, 70.0; range, 37 to 88 | Preop, 7.3; postop at 6 wk, 3.7 | Preop, 47.4; postop, 29.5 | NA | No major complications | 6 wk |
| Pao et al. [11] | 2009 | PCS | Microscopic decompressive laminoto-my | 53 (17 M, 36 F) | Average, 62.0; range, 36 to 86 | NA | Preop, 64.3±20.0; postop at last FU, 16.7±20.0 | Preop, 9.4±6.1; postop at last FU, 24.2±6.0 | UTI (2); dural tear (5); wrong level operation (2); transient neuralgia (4) | Average: 15.7 mo |
| Fu et al. [22] | 2008 | PCS | “Windows technique” laminoforaminotomy, traditional laminectomy | Windows: 76 (37 M, 39 F); laminectomy: 76 (33 M, 43 F) | Windows: average, 57; range, 47 to 70; laminectomy: average, 57; range, 45 to 73 | Windows: preop, 1.14±0.96; postop at last FU, 0.05±0.22; laminectomy: preop, 1.17±0.91; postop at last FU, 0.63±1.07 | Windows: preop, 39.26±6.54; postop at last FU, 0.37±0.96; laminectomy: preop, 39.76±6.94; postop at last FU, 3.37±8.55 | NA | Windows: dural tear (3); UTI (2); laminectomy: dural tear (2); UTI (2); degenerative spinal instability (6); repeat surgery with fusion (4) | Average: 40 mo |
| Ca vuşoğlu et al. [27] | 2007 | PCS | Unilateral laminectomy | 50 (21 M, 29 F) | Average, 69.81±15.15; range, 43 to 82 | Preop, 6.92±1.04; postop at last FU, 2.16±0.81 | Preop, 31.14±9.27; postop at last FU, 14.02±9.27 | NA | None | Average: 22.8 mo |
| Cho et al. [17] | 2007 | RCT | Marmot operation, traditional laminectomy | Marmot: 40 (16 M, 24 F); laminectomy: 30 (15 M, 15 F) | Marmot: average, 61.2±10.8; range: 52 to 77; laminectomy: average, 58.8±14.9; range, 42 to 70 | Marmot: preop, 6.45±2.60; postop, 2.38±1.89; laminectomy: preop, 7.07±1.49; postop, 4.00±2.00 | NA | Marmot: preop, 7.10±2.84; postop, 13.0±1.97; laminectomy: preop, 8.44±2.59; postop, 11.4±3.20 | No major complications | Marmot: average: 15.1 mo; laminectomy: average: 14.8 mo |
| Malmivaara et al. [23] | 2007 | RCT | Traditional laminectomy | 50 (11 M,39F) | Average, 63±9 | Preop, 6.7; postop at 2 yr, 2.9 | Preop, 33,0; postop at 2yr, 22.8 | NA | Dural tear (7); misplaced transpedicular screw (1); peridural hematoma (1); respiratory distress (1); repeat surgery (1) | 6 mo, 1 yr, 2 yr |
| Thome et al. [25] | 2005 | RCT | Bilateral laminotomy (group 1); unilateral laminotomy (group 2); laminectomy (group 3) | G1 : 40 (20 M, 20 F); G2: 40 (15 M, 25 F); G3: 40 (18 M, 22 F) | G1: average, 70±7; G2: average, 67±6; G3: average, 69±10 | G1 : preop, 7.5±2.3; postop at final FU, 2.3±2.4; G2: preop, 7.5±2.3; postop at final FU, 3.6±2.7; G3: preop, 7.5±2.3; postop at final FU, 4±1 | NA | NA | G1: incidental durotomy (2); G2: incidental durotomy (5); increased radicular deficit (2); repeat surgery (2); epidural hematoma (2); G3: incidental durotomy (8) repeat surgery (2); epidural hematoma (2); wound infection (1) | 3 mo, 6 mo, 12 mo |
| Ikuta et al, [12] | 2005 | Case series | Microendoscopic posterior decompression | 47 (23 M,24 F) | Average, 66; range, 31 to 88 | NA | NA | Preop, 14.3±4.1; postop at final FU, 24.9±3.2 | Du ral tear (4); intra-articular fracture (3); neurological deficit (7) | Average: 22 mo |
| Konno et al. [15] | 2000 | PCS | Traditional laminectomy | 42 | Average, 63 | Preop, 7.4; postop at 3 yr, 3.5 | NA | NA | No major complications | 1yr,3yr |
| Weiner et al, [24] | 1999 | PCS | Traditional laminectomy | 50 (29 M,21 F) | Average, 72; range, 49 to 88 | Preop, 8; postop, 2 | NA | NA | Dural tear (4) | 9 mo |
Patients were evaluated for change in preop and postop VAS, ODI, and JOA scores and postoperative complications.
VAS, Visual Analog Scale; ODI, Oswestry Disability Index; JOA, Japanese Orthopedic Association; FU, follow-up; PCS, prospective cohort study; M, male; F, female; NA, data was not reported for that category; Preop, preoperative; Postop, postoperative; MID, minimally invasive decompression; RCT, randomized controlled clinical trial; UTI, urinary tract infection; DVT, deep vein thrombosis; MI, microsurgical laminotomy; FI, full-endoscopic interlaminar bilateral decompression; ULBD, unilateral laminectomy for bilateral decompression; LSPD, lumbar spinous process splitting decompression; M-ULBD, modified unilateral laminotomy for bilateral decompression; w/o, without.
Table 2.
| Author | Year | Type of clinical trial | Type of interspinous device | Sample size | Age (yr) | VAS | ODI | Mortality/complications | FU |
|---|---|---|---|---|---|---|---|---|---|
| Nunley et al. [31] | 2017 | HCT | Superion | 89 | NA | Preop to postop % VAS leg change: 73% | Preop to postop % change: 73% | NA | 4 yr |
| Marcia et al. [32] | 2015 | Prospective cohort study | Percutaneous interspinous process spacer | 80 (39 M, 41 F) | Average, 70.4±8.7; range, 51 to 85 | Preop, 8.1±2; postop at 1 mo, 4.4±2 | Preop, 23.3±10; postop at 1 mo, 11.7±8.5 | Dislocation (1); spinous process fracture (2) | 1 mo, 1 yr, 3 yr |
| Patel et al, [34] | 2015 | HCT | Superion, X-stop | Superion: 190 (110 M, 80 F); X-stop: 201 (129 M,72 F) | Superion: average, 67±9; range, 47 to 88; X-stop: average, 66±10; range, 46 to 89 | NA | Superion: preop, 39±13; postop, 20±18; X-stop: preop, 40±12; postop, 18±15 | Superion (after 2 yr): back pain (49); leg pain (33); spinous process fracture (23); buttock/groin pain (10); X-stop (after 2 yr): back pain (61); leg pain (45); spinous process fracture (13); buttock/groin pain (12) | 6 wk, 3 mo, 6 mo, 12 mo, 18 mo, 24 mo |
| Puzzilli et al. [35] | 2014 | RCT | X-stop | Total: 542 (289 M, 253 F); X-stop: 422; non-surgical conservative treatment: 120 | Total: average, 63.6; range, 45 to 82 | X-stop: preop, 8.3; postop at 7 yr, 2.7 | NA | Total: spinous process fracture (16); cerebrospinal fluid leakages (9); superficial skin infection (13); device dislocation (18) | 1 mo, 6 mo, 1 yr, 2 yr, 3 yr, 4 yr, 5 yr, 6 yr, 7 yr |
| Patel et al, [33] | 2014 | HCT | Superion, X-stop | Total: 192 (150 M, 42 F); superion: 101; X-stop: 91 | Total: average, 67 | Superion: preop, 59±26; postop, 21±26; X-stop: preop, 55±26; postop, 21±25 | Superion: preop, 37±12; postop, 18±16; X-stop: preop, 39±12; postop, 20±16 | Superion: repeat surgery (18); X-stop: deep wound infection (2); repeat surgery (18) | 6 wk, 3 mo, 6 mo, 12 mo, 18 mo, 24 mo |
| Ploumis et al. [30] | 2012 | Case series | X-stop | 22 | Average, 64.5; range, 57 to 71 | Preop, 4.5±1.0; postop at last FU, 2.5±0.7 | Preop, 50.7±4.4; postop at last FU, 19.9±3.7 | No major complications | 2 yr |
| Van Meirhaeghe et al. [37] | 2012 | Prospective observational study | Aperius | 156 (78 M, 78 F) | Average, 64.8±11.9; range, 19 to 84 | Preop, 5.9±2.4; postop at 6 wk, 3.7±2.8 | NA | Removal of interspinous process decompression (14); spinous process fracture (1); back pain (1); hematoma (1); pain in extremity (1); spinal claudication (3); sciatica (1); arthralgia (6); lumbar spinal stenosis (3) | 6 wk, 12 mo |
| Siddiqui et al. [36] | 2007 | Prospective observational study | X-stop | 24 (12 M, 12 F) | Median, 71.5 | NA | Preop, 48; postop at 12 mo, 37 | Spinous process fracture (2) | 12 mo |
Patients were evaluated for change in preop and postop VAS and ODI scores and postop complications.
VAS, Visual Analog Scale; ODI, Oswestry Disability Index; FU, follow-up; RCT, randomized controlled clinical trial; NA, data was not reported for that category; Preop, preoperative; Postop, postoperative.
Table 3.
| Author | Year | Type of clinical trial | Type of decompression surgery and interspinous device | Sample size | Age (yr) | VAS | ODI | Mortality/complications | FU |
|---|---|---|---|---|---|---|---|---|---|
| Abdel Ghany et al.[43] | 2016 | Prospective clinical study | Traditional laminectomy, Coflex (21), X-stop (7) | Laminectomy: 25 (10 M, 15 F); ISP: 28 (22 M, 6 F) | Laminectomy: average, 51.8±8.2; ISP: average, 55±10.7 | Laminectomy: preop, 4.00±1.00; postop at last FU, 2.96±1.46; ISP: preop, 3.68±1.06; postop at last FU, 3.59±1.39 | Laminectomy: preop, 56.48±5.21; postop at last FU, 26.96±12.89; ISP: preop, 54.43±6.49; postop at last FU, 30.67±13.28 | Laminectomy: postop instability (3); dural tear (3); wrong level (1); meningitis (1); ISP: repeat surgery (6); spinous erosion (6); rotated X-stop (3); dural tear (1); wound infection (1); foot drop (1); wrong level (1) | 12 mo |
| Mohar et al.[42] | 2016 | RCT | Decompression: (posterior midline approach, flavectomy and laminotomy with possible additional partial facetectomy and/or foraminotomy, IDSDs | Decompression: 6 (2 M, 4 F); IDSD: 6 (4 M, 2 F) | Decompression: average, 51; range, 39 to 61; IDSD: average, 49; range, 36 to 55 | No statistically significant differences in pre- and postop ODI, VAS-lumbar and VAS-leg scores between groups | No statistically significant differences in pre- and postop ODI, VAS-lumbar and VAS-leg scores between groups | No major complications | Decompression: 188 day; IDSD: 196 day |
| Moojen et al. [38] | 2015 | RCT | Spinal bony decompression, unnamed ISP | Decompression: 79 (37 M, 42 F); ISP: 80 (49 M, 31 F) | Decompression: median, 64; range, 47 to 83; ISP: median, 66; range, 45 to 83 | Decompression: preop, 52; postop at 104 wk, 28; ISP: preop: 50; postop at 104 wk, 36 | NA | Decompression: epidural hematoma (2); dural tear (4); repeat surgery (6); ISP: spinous process fractures (3); wrong level (1); repeat surgery (23) | 2,4, 8,12, 26, 52, 104 wk |
| Lome et al.[40] | 2015 | RCT | MID, X-stop | MID: 41 (23 M, 18 F); X-stop: 40 (17 M, 23 F) | MID: average, 67±8.7; X-stop: average, 67±8.8 | NA | MID: preop, 33.8±2.5; postop at 2 yr, 18.4±2.6; X-stop: preop, 32.9±2.7; postop at 2 yr, 14.3±2.7 | MID: hematoma (3); postop cauda equine syndrome (1); dural tear (2); urine retention (1); repeat surgery (3); X-stop: spinous process fracture (1); late fracture (1); dislocation of X-stop (1); repeat surgery (3) | 6 wk, 3 mo, 1 yr, 2 yr |
| Marsh et al.[41] | 2014 | RCT | Priman/lumbosacral decompression,Wallis implant | Decompression: 30 (14 M, 16 F); Wallis: 30 (16 F, 14 M) | Decompression: average, 56.4±12.9; range, 34 to 76; Wallis: average, 59.6±13.4; range, 35 to 81 | Decompression: change from preop to last FU, 2.7±2.4; Wallis: change from preop to last FU, 3.5±3.4 | Decompression: change from preop to last FU, 10.6±19.3; Wallis: change from preop to last FU, 19.3±24.0 | No major complications | Average: 40 mo |
| Moojen et al.[39] | 2013 | RCT | Traditional decompression, FELIX | Decompression: 79 (37 M, 42 F); FELIX: 80 (49 M, 31 F) | Decompression: median, 66; range, 45 to 83; FELIX: median, 64; range, 47 to 83 | Decompression: preop, 49; postop at 52 wk, 31; FELIX: preop, 60; postop at 52 wk, 23 | NA | Decompression: epidural hematoma (2); dural tear (4); FELIX: visual disturbance (1); pseudoradicular leg pain (1); interspinous process fractures (3) | 2,4, 8,12, 26, 52 wk |
| Strömqvist et al. [44] | 2013 | RCT | Traditional decompression, X-stop | Decompression: 50 (26 M, 24 F); X-stop: 50 (30 M, 20 F) | Decompression: average, 71; range, 57 to 84; X-stop: average, 67; range, 49 to 89 | Decompression: preop, 60±26; postop at 24 mo, 23±29; Xstop: preop, 59±28; postop at 24 mo, 34±32 | NA | Decompression: dural injury (3); repeat surgery (3); X-stop: spinous process fracture (1) | 24 mo |
Patients were evaluated for change in preop and postop VAS and ODI scores and postop complications.
VAS, Visual Analog Scale; ODI, Oswestry Disability Index; FU, follow-up; M, male; F, female; ISP, interspinous process; Preop, preoperative; Postop, postoperative; RCT, randomized controlled clinical trial; IDSD, interspinous dynamic stabilization device; NA, data was not reported for that category; MID, minimally invasive decompression; FELIX, foraminal enlargement lumbar interspinous distraXion.






