|
 |
- Search
| Asian Spine J > Volume 10(1); 2016 > Article |
Abstract
Purpose
The purpose of this study was to study the relationship between prevalence of pyramidal signs and the severity of cervical myelopathy. The study is focused on patients having increased signal intensity in T2-weighted magnetic resonance imaging.
Overview of Literature
Cervical spondylotic myelopathy is the most common cause of spinal cord dysfunction in elderly population. It is the consequence of spondylotic changes leading to cervical cord injury with resulting clinical deficits. Diagnosis in such patients is made based on clinical and radiographic features. A patient must have both symptoms and signs consistent with cervical cord injury as well as radiographic evidence of damage to spondylotic cord.
Methods
Forty-six patients with complaint of cervical spondylotic myelopathy with increased signal intensity in T2-weighted magnetic resonance imaging were included in the study. The neurological finding of the patients was reviewed for the presence of pyramidal signs. The prevalence of each pyramidal sign was calculated and correlated to severity of cervical myelopathy. The motor function scores of the upper and lower extremities for cervical myelopathy set by the Japanese Orthopedic Association (motor Japanese Orthopaedic Association score, m-JOA) scores were used to assess severity of myelopathy.
The natural history of cervical spondylotic myelopathy (CSM) is variable, with some patients having a mild protracted course and others having progressive disability [1]. Cervical myelopathy is very difficult to be diagnosed especially in early stages due to unclear symptoms [2]. The syndrome of "numb, clumsy hands" has been described in patients with high compressive myelopathy between C-3 and C-5. Typical symptoms include loss of manual versatility accompanied with difficulty in writing; diffused, nonspecific arm weakness; and abnormal sensations. Lesions at levels C-5 to C-8 cause a syndrome of spasticity and proprioceptive loss in the legs. Such patients experience difficulty in walking and an unsteady feeling while walking; they often lose their balance and fall. Urinary frequency and urgency are common events experienced by the patients. A complete loss of bowel and bladder function is an end stage deficit, which is a rare occurrence [34].
Pyramidal signs such as hyperreflexia, Hoffmann reflex, clonus, and Babinski sign, are considered a corner stone in the clinical diagnostic process. However, patients with suspected myelopathy often lack these pathognomonic signs despite apparent symptoms and correlative cord compression on imaging studies. Such "atypical" neurological findings might be attributable to insufficient sensitivity of the pyramidal signs or to comorbidities that might affect neurological findings. Most of the patients did not express these pyramidal signs and the relation between the signs and severity of myelopathy is still indefinite [5].
Magnetic resonance imaging (MRI) remains the imaging modality of choice for CSM, even in an initial evaluation, because of its superior ability to show pathology of neural structures [6]. MRI allows for clear visualization of cord impingement or compression, and can be employed to accurately measure space within the spinal canal [7]. Increased signal intensity (ISI) at the level of cord damage, particularly on T2-weighted images, is due to inflammation, edema, ischemia, gliosis, or myelomalacia [8]. In fact, signal changes have been shown to be a fairly reliable indicator of irreversible cord damage [7]. Diffusion tensor MR imaging may enhance sensitivity for detecting CSM. Because of its ability to detect significant cord damage before the appearance of symptoms, it is currently being evaluated for its potential prognostic value [9]. Several studies have shown that patients with ISI were significantly more likely to show positive pyramidal signs [1011]. Although a majority of patients, who underwent surgery for cervical myelopathy appeared to have ISI, the literature has demonstrated varied incidence [1112131415].
The present retrospective study was performed to determine the sensitivities of the pyramidal signs in patients with cervical compressive myelopathy, with emphasis on the patients with ISI in preoperative T2-weighted MR images and to establish correlation between pyramidal signs and the severity of cervical myelopathy.
Clinical records of 71 patients operated between January 2010 and December 2013 for CSM were retrospectively reviewed. All patients had an ISI in their preoperative T2-weighted MR images as proven by radiological reports in patients' files. Twenty-five patients were suffering from one or more of the following comorbidities: cerebrovascular disease, diabetes mellitus, rheumatoid arthritis, peripheral neuropathy, hemodialysis, pacemaker, cerebral palsy, systemic bone disease, upper cervical lesion, spinal trauma, previous cervical surgery, previous or concomitant thoracic/lumbar surgery, and total joint replacement. The above-mentioned conditions constituted exclusion criteria and 25 patients suffering from either of such conditions were excluded from the current study. The remaining 46 patients (29 men and 17 women) with ISI and free from the previously mentioned comorbidities were included in this study.
This study was approved by the Institutional Review Board of Menoufia University. All patients provided written informed consent.
The preoperative MR images of the 46 patients were reviewed all over again by the author. All the 46 patients were found to have ISI in their MR images with substantial cord compression as evidenced by effacement of the subarachnoid space and indentation of the spinal cord. The concordance rate was 100% indicating good interobserver reliability. The preoperative neurological findings of all included patients were also reviewed. In addition, the signs were judged as positive when positive sign was present either unilaterally or bilaterally. Exaggerated patellar tendon reflex (PTR) was considered as positive. Ankle clonus, sustained clonus, was considered as positive. Hoffmann reflex flexion of the ipsilateral thumb and/or index finger was considered as positive. Babinski sign extension of the great toe was considered as positive.
The preoperative motor function of the patients was set by the Japanese Orthopedic Association (upper and lower motor Japanese Orthopaedic Association [m-JOA] score) as described earlier [5]. The overall sensitivity of each pyramidal sign was calculated.
A total of 46 patients (29 men and 17 women) met the study criteria. The mean age of patients at surgery was 51 years (range, 39–69 years). The follow-up period ranged from 2–48 months. The severity of preoperative motor dysfunction was measured based on the upper/lower m-JOA score (Table 1).
Hyperreflexia showed the highest sensitivity (89.1) followed by Hoffmann reflex (80.4), Babinski sign (56.5), and ankle clonus (39.1). The relationship between prevalence of the pyramidal signs and the severity of myelopathy as measured by the upper/lower m-JOA score using the Mann-Whitney U test was analyzed (Table 2).
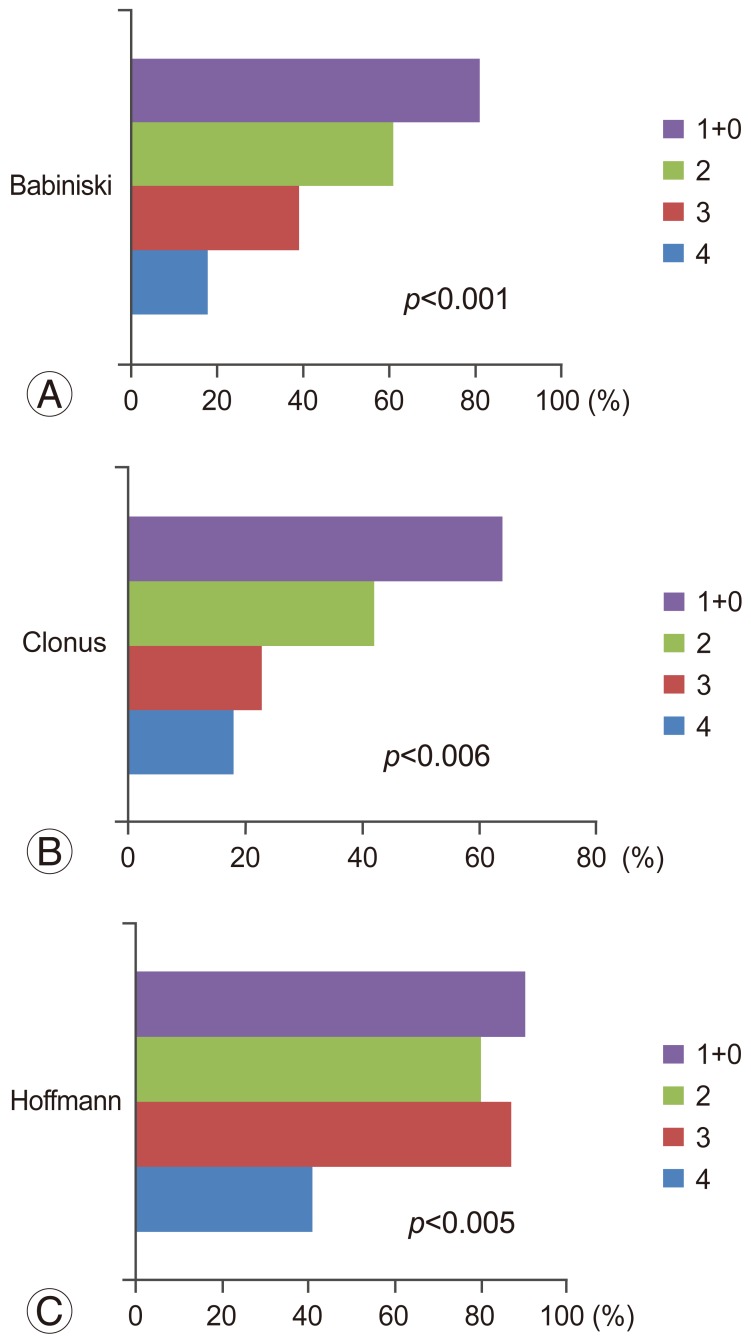
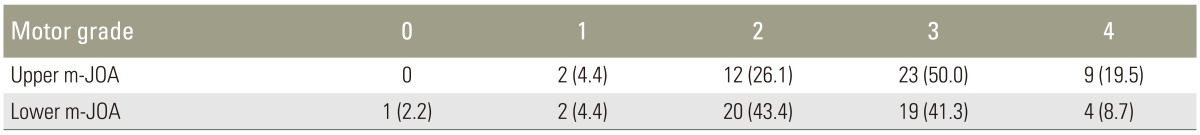
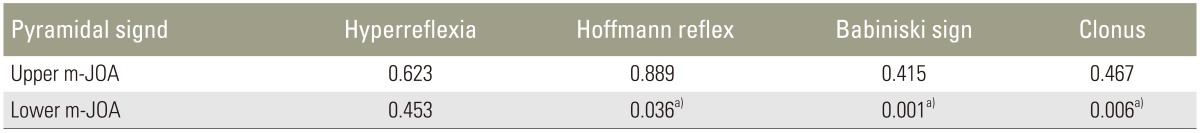
None of the pyramidal signs showed correlation with the upper m-JOA score. Statistical difference was observed in Babinski sign (p<0.001), ankle clonus (p<0.006), and Hoffmann reflex (p<0.05). Positive pyramidal sign was more frequently found with increasing severity of motor dysfunction in the lower extremities (Fig. 1).
In the present study, sensitivities of the pyramidal signs in patients with CSM with ISI in T2-weighted MRI were determined. Hyperreflexia showed the highest sensitivity (89.1%) followed by Hoffmann reflex (80.4%), Babinski sign (56.5%), and ankle clonus (39.1%). The present study also showed close correlation between prevalence of the pyramidal signs and increasing severity of myelopathy as measured by the lower m-JOA score. In contrast, no significant correlation was found with the upper m-JOA score.
In the current study, we focused on patients with ISI with respect to their MR images and excluded the patients with comorbidities that might complicate neurological findings. The clinical characteristics of subjects in the current study were almost equivalent to those reported in recent surgical series [15161718].
The prevalence of the pyramidal signs obtained in this study was almost consistent with those reported previously [10111920]. Considerable difference in sensitivity among these signs as depicted by Lunsford et al. was noted [20]. In line with most of the preceding studies, we found that sensitivities of exaggerated PTR and Hoffmann reflex were relatively high. Thus, this study recommends using PTR and Hoffmann reflex for screening myelopathic patients.
Rhee et al. [11] reported much lower sensitivity values for exaggerated PTR (33%), Hoffmann reflex (59%), Babinski sign (13%), and ankle clonus (13%) in patients, who showed no cord signal change. However, among 20 patients, who showed cord signal change the prevalence of PTR, Hoffmann reflex, Babinski sign, and ankle clonus increased to 45%, 80%, 15%, and 25%, respectively.
It was also found that prevalence of the pyramidal signs significantly correlated with the severity of myelopathy, especially with the severity of motor dysfunction in the lower extremities. This finding underscores the validity of these signs as a clinical indicator of impaired motor tract. In particular, the prevalence of Babinski sign is closely correlated with increasing severity of myelopathy as measured by the lower m-JOA score. Although varying prevalence values of Babinski sign (13%–54%) [10111920] have been reported in the literature, this discordance may be attributable to the varying severity of myelopathy.
Interestingly, prevalence of pyramidal signs exhibited no correlation with the severity of motor dysfunction in the upper extremities. The lack of correlation with the upper m-JOA score can be attributable to the following two reasons: first, the motor function of the upper extremities is affected not only by the severity of the cord compression but also by the level of the cord compression. Second, the motor function of the upper extremities might be maintained by compensatory mechanisms of the brain even with cord compromise [2122]. Probably for these reasons, the degree of cord compromise may not linearly correlate with the upper m-JOA score.
The association between prevalence of the pyramidal signs and the severity of myelopathy in the current study puts some limitations on the use of these pyramidal signs in early diagnosis of cervical myelopathy. The results of the current study demonstrate that prevalence of pyramidal signs was considerably low in mild cervical myelopathy therefore; using motor and somatosensory evoked potentials seems to be beneficial in such cases.
Having one examiner in the current study led to maximum validity of the data of the study concerning the neurological examination of the patients. Also, the sensitivity obtained in this study was estimated splendidly as it was recorded long time back by the examiner himself without having any prior information about the study design.
In patients with CSM, hyperreflexia showed the highest sensitivity followed by Hoffmann reflex, Babinski sign, and ankle clonus. For screening myelopathic patients, PTR and Hoffmann reflex may be useful because of high sensitivity. The prevalence of the pyramidal signs is closely correlated with increasing severity of myelopathy.
Conflict of Interest
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
1. McCormack BM, Weinstein PR. Cervical spondylosis: an update. West J Med 1996 165:43–51. PMID: 8855684.


2. Dillin WH, Watkins RG,Clinical syndromes in cervical myelopathy. Rothman RH, Simeone FA, editors. The spine. 3rd ed. Philadelphia: W.B. Saunders; 1992. p.560–570.
3. Good DC, Couch JR, Wacaser L. "Numb, clumsy hands" and high cervical spondylosis. Surg Neurol 1984 22:285–291. PMID: 6463840.


4. Voskuhl RR, Hinton RC. Sensory impairment in the hands secondary to spondylotic compression of the cervical spinal cord. Arch Neurol 1990 47:309–311. PMID: 2310314.


5. Chikuda H, Seichi A, Takeshita K, et al. Correlation between pyramidal signs and the severity of cervical myelopathy. Eur Spine J 2010 19:1684–1689. PMID: 20229121.



6. Demir A, Ries M, Moonen CT, et al. Diffusion-weighted MR imaging with apparent diffusion coefficient and apparent diffusion tensor maps in cervical spondylotic myelopathy. Radiology 2003 229:37–43. PMID: 14519868.


7. Hochman M, Tuli S. Cervical spondylotic myelopathy: a review. Internet J Neurol 2004 4:1–12.
8. Lee J, Koyanagi I, Hida K, Seki T, Iwasaki Y, Mitsumori K. Spinal cord edema: unusual magnetic resonance imaging findings in cervical spondylosis. J Neurosurg 2003 99:8–13. PMID: 12859052.


9. Ries M, Jones RA, Dousset V, Moonen CT. Diffusion tensor MRI of the spinal cord. Magn Reson Med 2000 44:884–892. PMID: 11108625.


10. Houten JK, Noce LA. Clinical correlations of cervical myelopathy and the Hoffmann sign. J Neurosurg Spine 2008 9:237–242. PMID: 18928217.


11. Rhee JM, Heflin JA, Hamasaki T, Freedman B. Prevalence of physical signs in cervical myelopathy: a prospective, controlled study. Spine (Phila Pa 1976) 2009 34:890–895. PMID: 19352222.


12. Holly LT, Moftakhar P, Khoo LT, Shamie AN, Wang JC. Surgical outcomes of elderly patients with cervical spondylotic myelopathy. Surg Neurol 2008 69:233–240. PMID: 18325426.


13. Morio Y, Teshima R, Nagashima H, Nawata K, Yamasaki D, Nanjo Y. Correlation between operative outcomes of cervical compression myelopathy and mri of the spinal cord. Spine (Phila Pa 1976) 2001 26:1238–1245. PMID: 11389390.


14. Suri A, Chabbra RP, Mehta VS, Gaikwad S, Pandey RM. Effect of intramedullary signal changes on the surgical outcome of patients with cervical spondylotic myelopathy. Spine J 2003 3:33–45. PMID: 14589243.


15. Yukawa Y, Kato F, Ito K, et al. Postoperative changes in spinal cord signal intensity in patients with cervical compression myelopathy: comparison between preoperative and postoperative magnetic resonance images. J Neurosurg Spine 2008 8:524–528. PMID: 18518672.


16. Iwasaki M, Okuda S, Miyauchi A, et al. Surgical strategy for cervical myelopathy due to ossification of the posterior longitudinal ligament: Part 1: Clinical results and limitations of laminoplasty. Spine (Phila Pa 1976) 2007 32:647–653. PMID: 17413469.


17. Sakaura H, Hosono N, Mukai Y, Oshima K, Iwasaki M, Yoshikawa H. Preservation of the nuchal ligament plays an important role in preventing unfavorable radiologic changes after laminoplasty. J Spinal Disord Tech 2008 21:338–343. PMID: 18600144.


18. Tsuji T, Asazuma T, Masuoka K, et al. Retrospective cohort study between selective and standard C3-7 laminoplasty: minimum 2-year follow-up study. Eur Spine J 2007 16:2072–2077. PMID: 17726618.



19. Chiles BW 3rd, Leonard MA, Choudhri HF, Cooper PR. Cervical spondylotic myelopathy: patterns of neurological deficit and recovery after anterior cervical decompression. Neurosurgery 1999 44:762–769. PMID: 10201301.



20. Lunsford LD, Bissonette DJ, Zorub DS. Anterior surgery for cervical disc disease. Part 2: Treatment of cervical spondylotic myelopathy in 32 cases. J Neurosurg 1980 53:12–19. PMID: 7411200.


21. Holly LT, Dong Y, Albistegui-DuBois R, Marehbian J, Dobkin B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine 2007 6:544–551. PMID: 17561743.



22. Mikulis DJ, Jurkiewicz MT, McIlroy WE, et al. Adaptation in the motor cortex following cervical spinal cord injury. Neurology 2002 58:794–801. PMID: 11889245.


Fig. 1
The figure demonstrates close correlation between prevalence of Babinski sign (A), clonus (B), and Hoffmann (C) with increasing severity of myelopathy as measured by the lower m-JOA score. m-JOA score, motor Japanese Orthopaedic Association score.