Association between Preoperative Urine Culture and Urinary Tract Infection after Spinal Surgery
Article information
Abstract
Study Design
This is a retrospective study.
Purpose
This study assessed risk factors accounting for urinary tract infections (UTIs) to determine whether preoperative asymptomatic UTI (aUTI) could be used to predict UTIs in patients after spinal surgery.
Overview of Literature
UTI is a spinal surgery complication that increases the incidence of surgical site infections. However, the risk factors for UTIs after spinal surgery remain unclear.
Methods
This study included 509 (mean age, 54.5 years; 239 males and 270 females) patients who underwent posterior spine surgery at the department of the current study. First, clean catch urine was collected, after which a urine culture was performed for all patients before surgery. Preoperative detection of the aUTI (>105 colony-forming units/mL) rate was then determined. Subsequently, risk factors for postoperative UTI were evaluated using logistic regression analysis with the following as independent variables: age, sex, obesity, diabetes, spinal cord tumor, the preoperative Japanese Orthopedic Association (JOA) score, JOA-bladder function, preoperative urine culture positivity, aUTI, preoperative Escherichia coli detection, the postoperative catheter placement period, instrumentation, number of surgical levels, surgery duration, and blood loss.
Results
The preoperative aUTI and postoperative UTI incidences were 8.1% and 4.1%, respectively. Furthermore, multivariate logistic analysis showed that the risk factor for postoperative UTI was preoperative aUTI (odds ratio, 4.234; 95% confidence interval, 1.532–11.702; p=0.005).
Conclusions
Preoperative aUTI is a risk factor for UTI in patients after spinal surgery.
Introduction
Urinary tract infections (UTIs) are a common disease that is more likely to occur in older patients and women [1]. Various complications occur after spinal surgeries, including UTIs (2.6%), dural injuries (2.2%), surgical site infections (SSIs) (2.3%), and hematoma (0.7%) [2], and postoperative spinal surgery infections have notably been associated with increased rates of morbidity, mortality, length of hospital stay, and financial burden [3]. Bohl et al. [4] reported that the risk factors for UTI after lumbar fusion were older age, female sex, functional dependency, malnourishment, and diabetes [4]. Subsequently, postoperative UTIs increase SSIs [5]. Moreover, several reports have demonstrated an association between preoperative urinary tract colonization and the later development of SSI following instrumented spinal surgery [6,7]. Currently, the incidence of preoperative asymptomatic UTI (aUTI) and postoperative UTI in patients remain unknown. Furthermore, the association between preoperative urinary bacteria and postoperative UTI remains unclear. Therefore, this study assessed the risk factors for UTIs and determined whether preoperative aUTI could predict UTIs in patients after spinal surgery.
Materials and Methods
1. Subjects
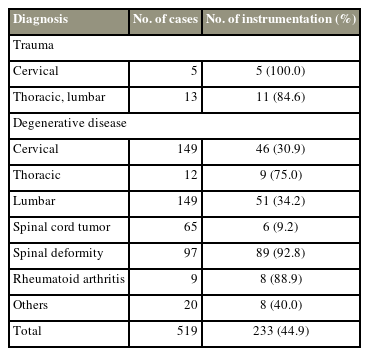
This retrospective cohort study enrolled 519 patients who underwent spinal surgery between 2014 and 2020 (Fig. 1). However, 10 patients with preoperative pyogenic spondylitis and septic wound conditions were excluded. Thus, only 509 patients were included in this study, comprising 239 men and 270 women (mean age, 54.5 years old; range, 6–86 years old). The incidence of preoperative diagnoses and instrumentation is shown in Table 1. Subsequently, 233 patients (44.9%) underwent spinal instrumentation surgery, after which demographic data between diagnosis and sex were compared to distinguish between diagnosis and sex characteristics. All patients provided written informed consent before the assessment. Furthermore, the Institutional Review Board of Hirosaki University Graduate School of Medicine approved this study (2019-1038).
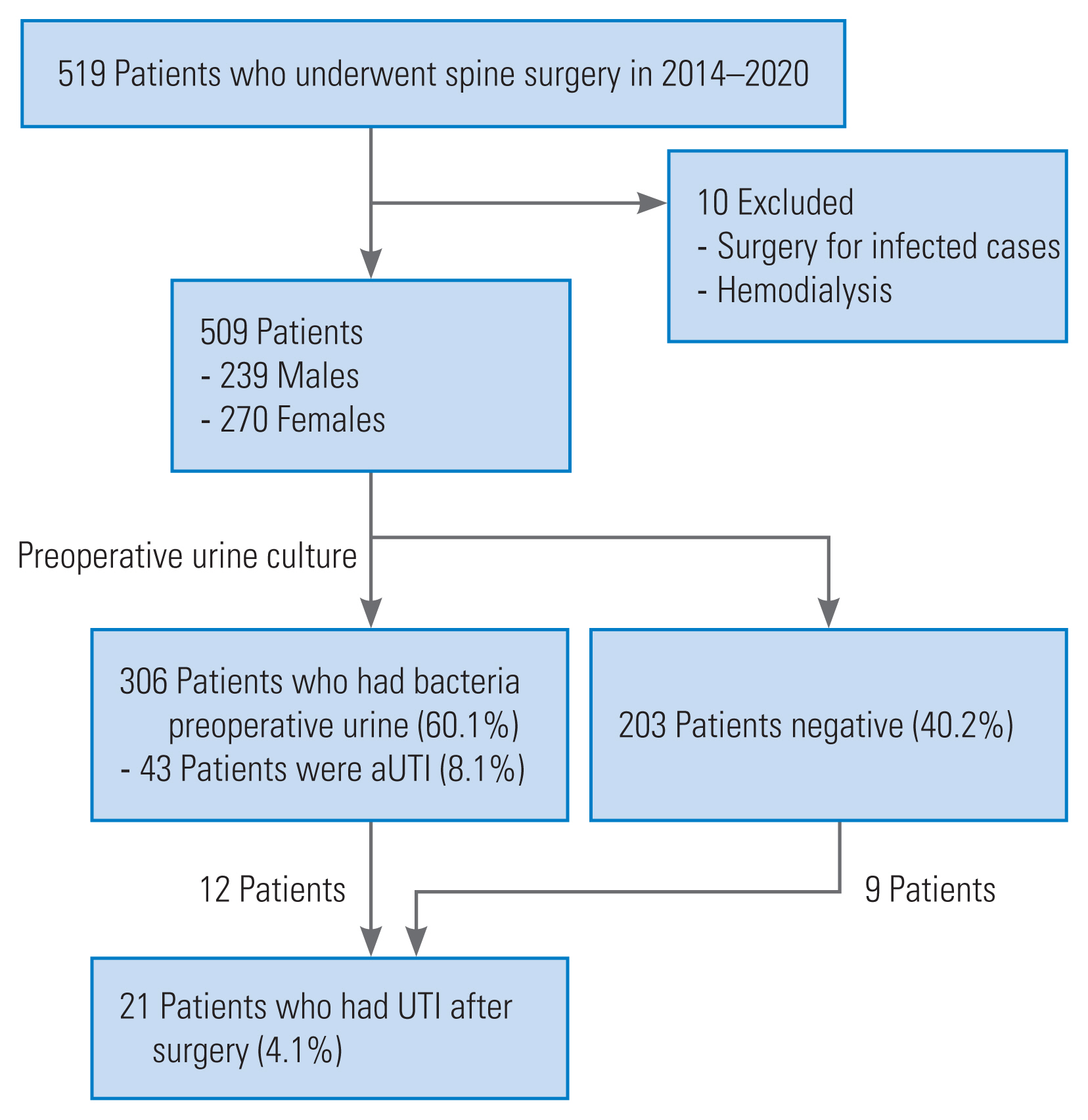
The flow diagram of this study. A total of 509 patients underwent posterior spinal surgery at our department (239 men and 270 women). Preoperative urine culture and asymptomatic urinary tract infection (aUTI) detection rates were 60.1% (306 cases) and 8.1% (43 cases), respectively. Twenty-one patients (4.1%) had urinary tract infection (UTI).
2. Perioperative protocol
Perioperative management was performed as described by Numasawa et al. [8] in 2015. Smokers were instructed to cease smoking for at least 4 weeks before the surgery. Also, while patients with diabetes and hemoglobin A1C values >7% underwent glycemic control before surgery, those aged >60 years old maintained controlled blood glucose levels for 1–2 weeks. Furthermore, the surgery was postponed and performed after UTI treatment when symptomatic UTI symptoms (e.g., fever or pain during urination), were observed. Moreover, prophylactic antibiotics were administered via intravenous (IV) drip infusions during the preoperative and intraoperative periods although antibiotics were not used in patients with preoperative aUTI. Notably, an IV infusion drip to avoid allergies was used [9] because the altered effectiveness of the shot has been reported [10]. Cefazolin (based on weight) was then administered as a first-line treatment unless the patient had a significant history of drug allergies (e.g., anaphylactic shock, systemic skin eruption, or toxic liver dysfunction). Preoperative antibiotics were administered within 30 minutes before skin incision. However, an additional dose of antibiotics was administered intraoperatively every 4 hours. No additional antibiotics were administered postoperatively. Finally, the urinary catheter was inserted after general anesthesia for general care and removed 3 days after surgery. However, placement of the urinary catheter was continued if a urinary catheter was needed due to difficulty in urinating or delaying mobilization.
3. Preoperative urine culture analysis
Urine cultures of all patients were sent to the microbiology laboratory of the authors’ institution 1–2 days before surgery. In all cases, the colony-forming units (CFU) of the urine culture was examined. All cultures were then grown and monitored for 7 days. Urine cultures with a bacterial count >102 CFU/mL were defined as positive. Patients without urinary symptoms but a bacterial urine count of >105 CFU/mL were classified as aUTIs [6]. If the urine cultures were positive, their sensitivities were subsequently examined for various antibiotics. To distinguish the characteristics of the patients with aUTI, the demographic data between the aUTI and non-aUTI groups were then compared.
4. Diagnosis and management of postoperative urinary tract infection
As previously described [5], UTI was diagnosed by the presence of consistent clinical symptoms and/or fever without infectious foci, leukocyturia, and positive urine culture with a bacterial count of 105 CFU/mL. Antibiotics were used based on sensitivity studies in cases where bacteria were detected in the preoperative urine culture. However, broad-spectrum antibiotics were administered if bacteria were undetected in the preoperative urine culture. The demographic data between the preoperative UTI and nonUTI groups were then compared to distinguish the characteristics of the patients with UTIs. Multivariate logistic regression analysis was finally performed to identify the risk factors for UTIs.
5. Assessment of neurological function
The severity of the clinical symptoms was evaluated using the Japanese Orthopedic Association (JOA) score for cervical spine surgery, comprising six subscores: upper extremity motor including deltoid, biceps, and brachioradialis; lower extremity motor; upper extremity sensory; lower extremity; trunk sensory; and bladder function (BF). Subsequently, the JOA-BF classified dysuria into four stages: point 3, normal BF; point 2, urinary retardation and pollakiuria; point 1, urinary retention, dribbling, thin stream, and incomplete continence; and point 0, urinary retention and incontinence.
6. Statistical analysis
The IBM SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA) software was used for statistical analyses. The chi-square test, Fisher’s exact test, and residual analysis compared the categorical variables, and the T-test was used for continuous variables. In addition, age, sex, obesity (body mass index >25 kg/m2), diabetes, spinal cord tumor, lumbar disease, preoperative JOA score, JOA-BF, preoperative urine culture positivity, presence of aUTI, preoperative Escherichia coli detection, postoperative catheter placement period, instrumentation, number of surgical levels, surgical duration, and blood loss were also examined. All statistical tests were two-tailed, and the threshold for statistical significance was set at p<0.05. Subsequently, sequential univariate and multivariate logistic regression analyses were performed to identify independent risk factors for UTIs. After the univariate analysis, the independent multivariate analysis variables included factors with p-values <0.05.
Results
1. Preoperative urine bacterial culture and incidence of preoperative asymptomatic urinary tract infection
Of the 509 patients, bacteria (>102 CFU/mL) were detected in the preoperative urine cultures of 306 patients (60.1%) (Fig. 1), and aUTI (>105 CFU/mL) was diagnosed in 43 patients (8.1%). Bacterial growth frequencies are shown in Table 2. Commonly detected strains include methicillin-sensitive coagulase-negative Staphylococci (MSCNS, 24.8%), Streptococcus (20%), E. coli (9.6%), and Enterococcus, and Corynebacterium (8.0%). However, the most frequently detected strains in the cultures of aUTI cases were E. coli (35.7%) and Enterococcus (26.2%). Seventeen cases (39.5%) of aUTI were cefazolin-resistant.
2. Comparison between asymptomatic urinary tract infection and non-asymptomatic urinary tract infection groups
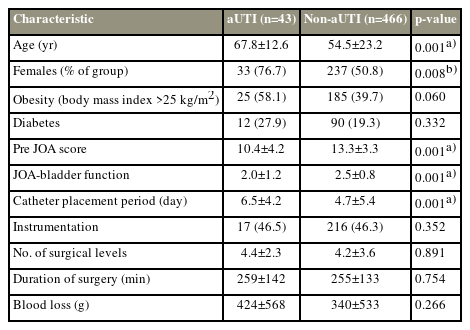
The demographics between the aUTI and non-aUTI groups were compared (Table 3). The prevalence of the female sex in the aUTI group was significantly higher than that in the non-aUTI group (p=0.008). Results also showed that while age (p=0.001) was significantly higher in the aUTI group than in the non-aUTI group, the preoperative JOA score (p=0.001) and JOA-BF (p=0.001) of the aUTI group were significantly lower than those of the non-aUTI group.
3. Incidence and treatment of postoperative urinary tract infection
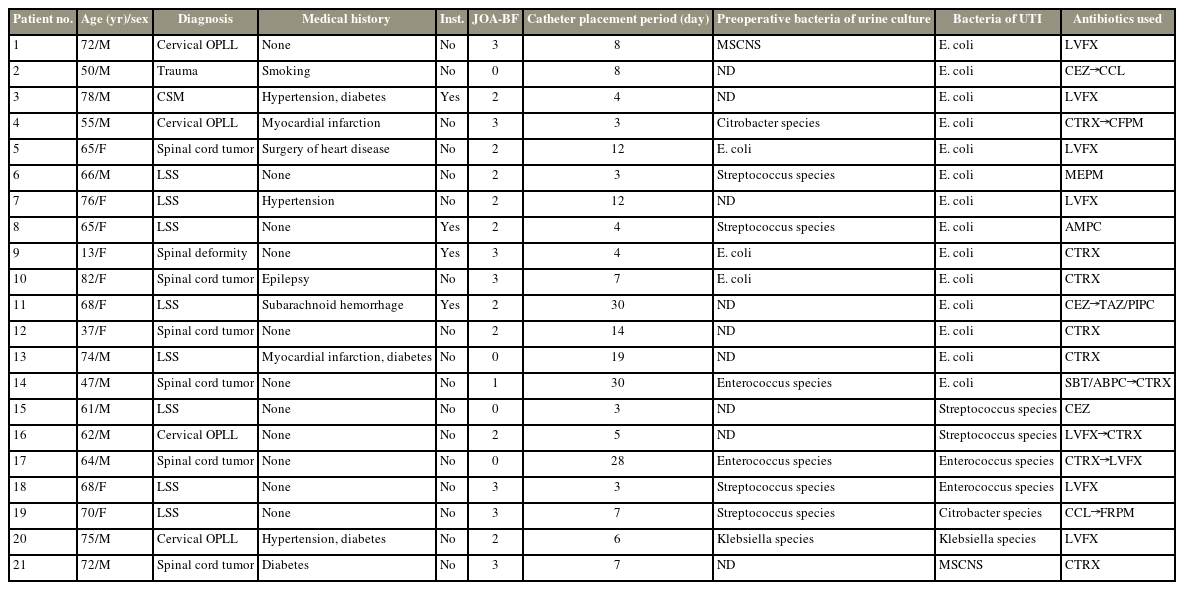
Twenty-one patients (4.1%) had UTIs after spinal surgery (Fig. 1). UTI cases are presented in Table 4. Among these patients, 1 (1.7%) was <18 years. Results also showed that 4 (19.0%) of 21 patients underwent spinal instrumentation surgery. The causative bacteria of UTI were E. coli in 14 cases, Enterococcus in two cases, Streptococcus in two cases, and Citrobacter species/MSCNS in one case each. However, similar bacteria were grown in the preoperative and postoperative urine cultures in five of the 21 cases (23.8%). Notwithstanding, all patients with UTIs were treated with antibiotics based on culture sensitivity studies.
4. Comparison between males and females
The demographics of the male and female patients were compared (Table 5). The prevalence of aUTI in women was significantly higher than that in men (p=0.011). However, the preoperative JOA score of men was significantly lower than that of women (p=0.008).
5. Characteristics of diagnostic findings
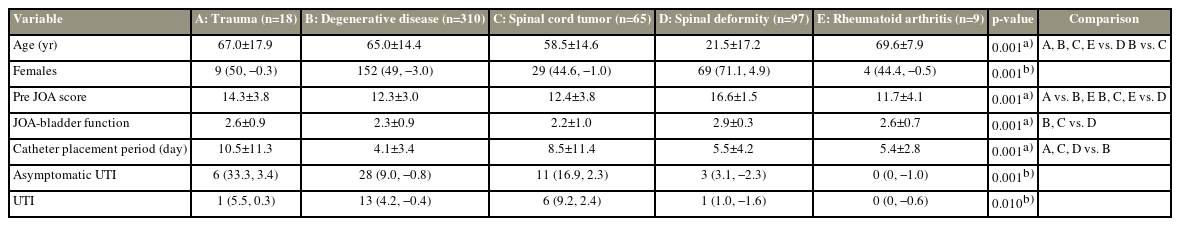
The demographics of those with trauma, degenerative disease, spinal cord tumor, spinal deformity, and rheumatoid arthritis were compared (Table 6). The preoperative JOA score and JOA-BF of spinal deformity were lower than those with degenerative diseases and spinal cord tumors (all p=0.001). Results also showed that the catheter placement period for trauma and spinal cord tumors was longer than for the degenerative disease (p=0.006 and p=0.001, respectively). Additionally, the prevalence of aUTI in the trauma and spinal cord tumor (p=0.001; residual trauma, 3.1; spinal cord tumor, 2.3), including the prevalence of UTI in the spinal cord tumor (p=0.010; residual, 2.4), was high.
6. Risk factors for postoperative urinary tract infection
Univariate logistic regression analysis showed a significantly positive association between the preoperative JOA score (B=−0.135; odds ratio [OR], 0.874; 95% confidence interval [CI], 0.787–0.970; p=0.012), JOA-BF (B=−0.426; OR, 0.653; 95% CI, 0.442–0.964; p=0.032), spinal cord tumor (B=0.560; OR, 3.046; 95% CI, 1.136–8.146; p=0.025), the prevalence of aUTI (B=1.884; OR, 6.579; 95% CI, 2.581–16.771; p=0.001), and the catheter placement period (B=0.064; OR, 1.066; 95% CI, 1.025–1.119; p=0.001) (Table 7). Subsequently, multivariate logistic analysis showed that the risk factor for postoperative UTI was preoperative aUTI (B=1.449; OR, 4.234; 95% CI, 1.532–11.706; p=0.005) (Table 7).
Discussion
The novelty of this study is that it clarifies the demographics of patients with aUTI, postoperative UTI, and their associations. Preoperative aUTI, diagnosed in patients with cultures having a bacterial count >105 CFU/mL, was detected in 8.1% of the patients, with E. coli being the most detected bacterial species. Furthermore, while the incidence of UTI after surgery was 4%, the risk factor for UTI after spinal surgery was the presence of preoperative aUTI.
This study also showed that preoperative aUTI was present in 8.1% although urine cultures were positive in 60.1% of patients. Bacteriuria is usually present in at least 20% of women and >10% of men >65 years old [11]. Accordingly, Yoshida et al. [12] in 2019 performed bladder urine culture in patients scheduled for urological surgery and detected bacteria in 40.8% of the patients. In many previous studies, the most common causative bacteria of UTI were E. coli [13]. Similarly, the most frequently detected bacteria in preoperative urine cultures were MSCNS with a bacterial count >102 CFU/mL and E. coli with a bacterial count >105 CFU/mL. E. coli, being the most common in patients with aUTI, is consistent with previous studies. However, a probable reason for MSCNS being the most detected species is that it is commonly found in skin flora and is likely to contaminate the sample during urination.
The demographics of patients with aUTI showed a high prevalence of females, low JOA-BF, and a longer catheter placement period. The aUTI mechanism may be explained as follows: first, women are generally more likely to develop UTI [14] because their thick and short urethra facilitates the entry of bacteria into the bladder. The second is the presence of a neurogenic bladder (NGB). NGB is a disease involving the loss or absence of normal bowel function due to nerve injury, neurological disease, or congenital nervous system defects [15]. Therefore, in some reports, 80% of spinal cord injury cases develop NGB [16] and are prone to repeated UTIs [17]. Third, 80% of UTI cases in hospitalized orthopedic patients are related to urinary catheterization [18], causing an increased incidence of 5%–7% with each additional day of urinary catheter placement [19]. Moreover, the catheter placement period was significantly different in the simple regression analysis except during diagnosis. Consequently, a strategy to prevent UTIs is to reduce the catheter placement period.
Additionally, this study identified 21 patients (4.1%) with UTIs after spinal surgery. The incidence of UTIs after spinal surgery in studies ranged from 1.77% to 19.1% [4–6]. However, these reports investigated the elderly and adults. Nevertheless, only a few studies have been published on UTIs after spinal surgery in children although pediatric UTIs are common, with an overall prevalence ranging from 2% to 8% throughout childhood [20]. Accordingly, the results of the current study revealed that only one UTI patient was aged <18 years old. Therefore, the incidence of UTI in this study was similar to that reported in previous studies in children and adults.
Six of the 21 patients (28.6%) with UTIs had spinal cord tumors. In this study, the incidence of UTI in patients with spinal cord tumors was higher than that in patients with other diseases (9.2%; p=0.01; residual, 2.4). Focusing on the urinary catheter period, patients with trauma and spinal cord tumors underwent long-term urinary catheter placement because their neurological deficits induced a NGB. While patients with trauma have a high preoperative JOA score, those with spinal cord tumors have a low preoperative JOA score. Consequently, patients with spinal cord tumors had more UTIs than that with other diagnoses. Based on these results, the presence of spinal cord tumors was added as a confounding factor. Univariate logistic regression analysis revealed that the prevalence of spinal cord tumors was significantly associated with UTI (B=0.560; OR, 3.046; 95% CI, 1.136–8.146; p=0.025), whereas multivariate logistic analysis revealed that it was not significantly associated with UTI. Furthermore, women are not at risk of developing UTIs. However, since the risk of UTI was aUTI, and women had more aUTI than men, the prevalence of UTI was predicted to be high in women. Contrastively, the results of the current study showed no significant difference between the sexes in postoperative UTIs. Studies have also reported that while the aUTI group had a longer urinary catheter placement period and a lower JOA-BF than the non-aUTI group, the aUTI group had a low JOA-BF, and the patients were more likely to have difficulty urinating. Therefore, based on previous studies, the urinary catheter placement period was longer in the aUTI group. These results indicate that patients with aUTI are at risk of developing UTI.
Postoperative UTI increases the incidence of SSIs although postoperative UTI control is important to prevent SSI [5]. Previously, Nunez-Pereira et al. [5] reported that patients who underwent spinal surgery had the same causative bacteria for UTI and SSI and were also more likely to have an SSI. However, administering antibiotics based on preoperative urine culture reduces the incidence of SSIs [4]. Accordingly, the current study showed that patients with aUTI were at an increased risk of developing UTI. Therefore, preoperative intervention in patients with aUTI may reduce the incidence of UTI and SSI. Investigations also revealed that the proportion of the same bacteria detected in both preoperative and postoperative urine cultures was only 23.8%. Antibiotics targeting preoperative urine cultures may also be ineffective in several cases. Contrastively, performing a urine culture in all postspinal surgery cases is not recommended from a medical and economic viewpoint. Nevertheless, as previously reported [5], preoperative urine cultures are recommended for patients susceptible to UTIs. Hence, using a urine culture, this study showed that while patients with preoperative aUTI were at a high risk of developing UTI, those with aUTI were females and had a low JOA score and a low JOA-BF.
Several limitations should be addressed despite this study’s novel findings. First, the medical history of the included patients with UTIs was not investigated. Patients with a history of aUTI have an increased risk of developing UTI [21]. Therefore, the association between the aUTI history of patients and UTI after spinal surgery should be investigated in the future. Second, dysuria was classified only based on the JOA score of the cervical spine. Thus, differentiating postoperative UTI dysuria from preoperative spinal or cauda equina-induced dysuria was impossible. Consequently, distinguishing between dysuria due to cauda equina symptoms and dysuria due to UTI was difficult. Based on these findings, this evaluation method has been proposed as unsuitable. Hence, this study evaluated all spinal patients, including those with thoracic spine disease, using the JOA score of the cervical spine, and some patients may not have been properly evaluated. Third, only a few UTI patients were noted [22], which may have impaired the accuracy of the logistic analysis results. Fourth, preoperative antibiotics were not selected based on the preoperative urine culture results. Based on these results, UTIs could have been prevented using antibiotics. Thus, understanding the risk of UTIs and performing postoperative management is also important.
Conclusions
Although the incidences of preoperative aUTI and postoperative UTI were 8.1% and 4.1%, respectively, preoperative aUTI was a risk factor for UTI. Subsequently, preoperative urine cultures were recommended to be performed for patients at a high risk of aUTI, and culture sensitivity-based antibiotics should be used in patients with a bacterial count >105 CFU/mL in preoperative urine cultures and those scheduled for long-term catheter placement.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
TN and GK wrote and prepared the manuscript, and all of the authors participated in the study design. All authors have read, reviewed, and approved the article.