In Vertebral Hemangiomas with Neurological Deficit, Is a Less Extensive Approach Adequate?
Article information
Abstract
Study Design
This was a retrospective study.
Purpose
To analyze the surgical and neurological outcomes following surgical decompression in patients with aggressive vertebral hemangioma (AVH) presenting with neurological deficit and to determine whether a less extensive approach is appropriate.
Overview of Literature
AVHs are a rare subset of benign vascular tumors frequently presenting with neurological deficit because of spinal cord compression. Though the results of surgical management have improved over time, there is a lack of consensus on the ideal management in this group of patients.
Methods
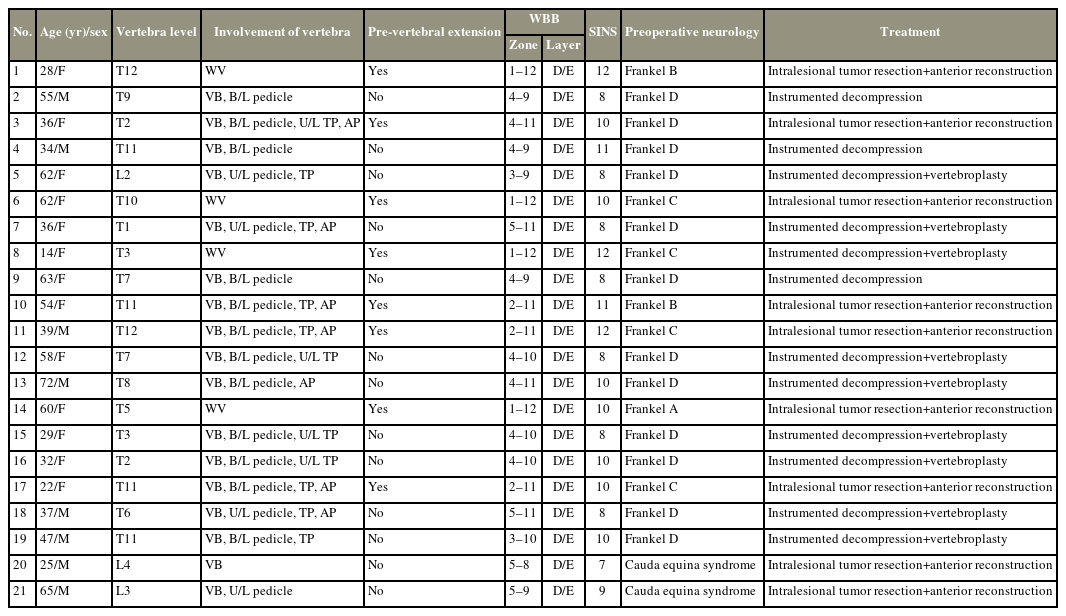
Twenty-one patients who underwent surgery for AVH between 2009 and 2018 were analyzed. Demographic and clinical details of patients were retrieved from hospital information system. Imaging information (i.e., radiography, computed tomography, magnetic resonance imaging) of all patients was accessed and analyzed in picture archiving and communication system. Tumor staging was performed using Enneking and Weinstein–Boriani–Biagini classifications and Spinal Instability Neoplastic Score. At follow-up, neurological and radiological evaluations were performed.
Results
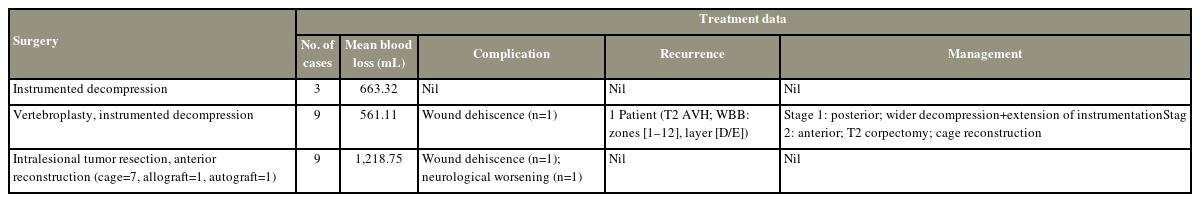
Twenty-one patients (13 [61.9%] females and 8 [38.1%] males) were included with a mean age of 44.29 years (range, 14–72 years). All patients in the study had neurological deficit. Back pain was present in 80.9% of patients. Mean duration of symptoms was 4.6 months (range, 1 day to 10 months). Most common lesion location was thoracic spine (n=12), followed by thoracolumbar (D11–L2; n=7) and lumbar (n=2) regions. Ten patients had multiple level lesions. All patients underwent preoperative embolization. Nine patients underwent intralesional spondylectomy with reconstruction; another nine patients underwent stabilization, decompression, and vertebroplasty; three patients underwent decompression and stabilization. Neurology improved in all patients, and only one case of recurrence was noted in a mean follow-up of 55.78±25 months (range, 24–96 months).
Conclusions
In AVH, good clinical and neurological outcomes with low recurrence rates can be achieved using less extensive procedures, such as posterior instrumented decompression with vertebroplasty and intralesional tumor resection.
Introduction
Vertebral hemangiomas (VHs) are benign vascular hamartomas detected incidentally during radiological evaluation with a reported incidence of 10%–12% [1]. Based on the Enneking classification, VHs are classified into three types: latent, active, and aggressive [2]. Latent lesions (Enneking stage 1) have mild bony destruction without symptoms, whereas active lesions (Enneking stage 2) present with pain because of bony destruction. Aggressive VHs (AVHs) (Enneking stage 3) are lesions with bony destruction and epidural soft tissue extension that are seen in 1% of affected patients [3,4]. Among all patients with AVHs, 55% present with pain, whereas the remaining 45% present with compressive myelopathy or neurological deficits, and therefore, aggressive management is indicated in this subgroup of patients [3].
Many treatment modalities have been applied over the years, including radiotherapy [5,6], vertebroplasty [7–10], intralesional spondylectomy [11,12], and en bloc spondylectomy [13]. AVHs without neurological symptoms are often treated with vertebroplasty [14] or radiotherapy [15]. In patients with AVH presenting with neurological involvement, the use of radiotherapy alone has been associated with a high risk of pathological fracture and recurrence [16,17]. Hence, surgical intervention is the treatment of choice, which is preferably preceded by preoperative embolization [18,19]. Surgical options for AVHs include en bloc spondylectomy [13] with anterior reconstruction, intralesional spondylectomy with reconstruction [12], and posterior instrumented decompression with or without vertebroplasty [20]. Song et al. [21] reported that AVHs treated with en bloc spondylectomy showed no local recurrence but was associated with a significant blood loss. In contrast, in a study by Wang et al. [20] involving 39 patients with AVH, less extensive procedures, such as posterior instrumented decompression and vertebroplasty, resulted in good patient outcomes with only one case of recurrence.
Despite all these treatment strategies, no clear consensus exists in the literature regarding the management of AVH because of the rarity of these lesions. With this in the background, we conducted a retrospective analysis of 21 consecutive cases of AVH operated at Ganga Medical Centre and Hospitals to enumerate treatment strategies.
Materials and Methods
1. Patient and study design
A retrospective study of patients with AVH treated at our institution between January 2009 and January 2018 was conducted after obtaining the Institutional Review Board of Ganga Medical Centre and Hospitals pvt. Ltd. (IRB approval no., 2021/02/14). Informed consent was obtained from all individual participants included in the study. In this study, 21 patients with a minimum follow-up duration of 2 years were identified. The data were collected using the hospital information system, operative registry, and picture archiving and communication system (PACS). The patients’ demographic details, clinical presentation, neurological status, surgical procedure, intraoperative blood loss, complications, outcomes, and recurrence were noted and analyzed. Additionally, the patients’ neurological status was assessed using the Frankel score.
Radiographs, computed tomography, and magnetic resonance imaging (MRI) were accessed and analyzed in the hospital PACS. The extent of vertebral involvement was noted along with paraspinal and epidural extensions and classified according to the Weinstein–Boriani–Biagini (WBB) classification system [2]. Tumor staging was performed using the Enneking classification and Spinal Instability Neoplastic Score (SINS) [22]. All patients underwent preoperative embolization the day before surgery. All surgeries were performed by three senior spine surgeons at our institution (i.e., S.R., A.P.S., and R.M.K.), and the choice of the procedure was based on the severity of neurological deficit and the preference of the surgeon. Intraoperative neuromonitoring was used. The patients were followed up at 3, 6, and 12 months postoperatively and then annually. At each visit, neurological and radiological evaluations were performed. MRI was obtained at the 3-month follow-up.
2. Treatment protocol
Our treatment strategy has changed over the last few years. All patients included in this study underwent preoperative embolization the day before surgery. Initially, we performed decompressive laminectomy with stabilization in few patients with nonprogressive or mild neurological deficits (Frankel D). However, now, our preferred treatment is to perform posterior instrumented decompression with vertebroplasty in patients with signs of early myelopathy (hyperreflexia) or minimal neurological deficit (Frankel D). In these patients, following posterior instrumentation with pedicle screws, laminectomy was performed at the level of the lesion to decompress the neural elements. Then, methyl methacrylate bone cement was injected into the vertebral lesion through a transpedicular approach under vision, followed by wide decompression, which included a single pediculectomy.
However, in patients with progressive or significant neurological deficits with features of myelopathy, instrumented decompression with intralesional tumor resection was performed as a staged procedure. In stage I, decompression and posterior stabilization with pedicle screw instrumentation were performed. In stage II, intralesional excision of the tumor was performed using an anterior approach. En bloc tumor resection, as an index surgery, was not performed in any patient.
Results
1. Demography
In this study, 21 patients with a mean age of 44.29 years (range, 14–72 years) were included. Eight patients (38.1%) were males and 13 (61.9%) were females with a male-to-female ratio of 1:1.6. Two female patients were in their postpartum period (12 and 28 weeks postpartum).
2. Symptomatology
All 21 patients (100%) had neurological deficits (Frankel A-1, Frankel B-2, Frankel C-4, and Frankel D-12) at presentation. Two patients presented with features of cauda equina syndrome. Back pain was present in 17 patients (80.95%). The mean duration of symptoms before presentation to the hospital was 4.61 months (range, 1 day to 10 months).
3. Imaging and staging
The most common lesion site was the thoracic spine (n=12), followed by the thoracolumbar (T11–L2; n=7) and lumbar (n=2) regions. Among the 21 patients, 10 (10/21) also had asymptomatic (Enneking stage 1) latent lesions at multiple levels.
All patients had vertebral body involvement with posterior cortical breach and epidural extension. An additional paravertebral extension was observed in eight patients. Pedicle involvement was observed in 20 patients (unilateral [n=3] and bilateral [n=17]). The involvement of the entire vertebrae (anterior and posterior elements) was noted in four patients. According to the WBB classification, all patients had layer D/E involvement. The mean SINS score was 9.52 (range, 7–12). The WBB staging and SINS scores of all patients are enumerated in Table 1.
4. Treatment, complications, and recurrence
As the clinico-radiographic features were suggestive of aggressive hemangiomas, preoperative biopsy was not performed in our patients. All patients underwent preoperative embolization. In this study, the overall mean intraoperative blood loss was 857 mL (range, 470–1,700 mL). Histopathology confirmed the diagnosis of aggressive hemangioma in all patients.
Three patients underwent posterior instrumented decompression with an average blood loss of 663.32 mL (range, 550–750 mL). Nine patients underwent vertebroplasty with instrumented decompression with an average blood loss of approximately 561.11 mL (range, 470–630 mL) (Fig. 1). Intralesional tumor resection with anterior reconstruction was performed in nine patients with an average blood loss of 1,218.75 mL (range, 800–1,700 mL) (Fig. 2). One patient had neurological worsening following surgery, which recovered during the follow-up. Overall, two patients in this study (one patient underwent vertebroplasty with instrumented decompression, and one patient underwent intralesional tumor resection and anterior reconstruction) had wound dehiscence requiring resuturing.

(A, B) Anteroposterior and lateral radiographs showing a lesion at T7. (C, D) Computed tomography images (sagittal and axial) showing “Polka dot” appearance. (E, F) Magnetic resonance imaging sequences showing epidural extension causing cord compression. (G, H) Postoperative radiographs after T5–T9 posterior instrumented decompression and T7 vertebroplasty.
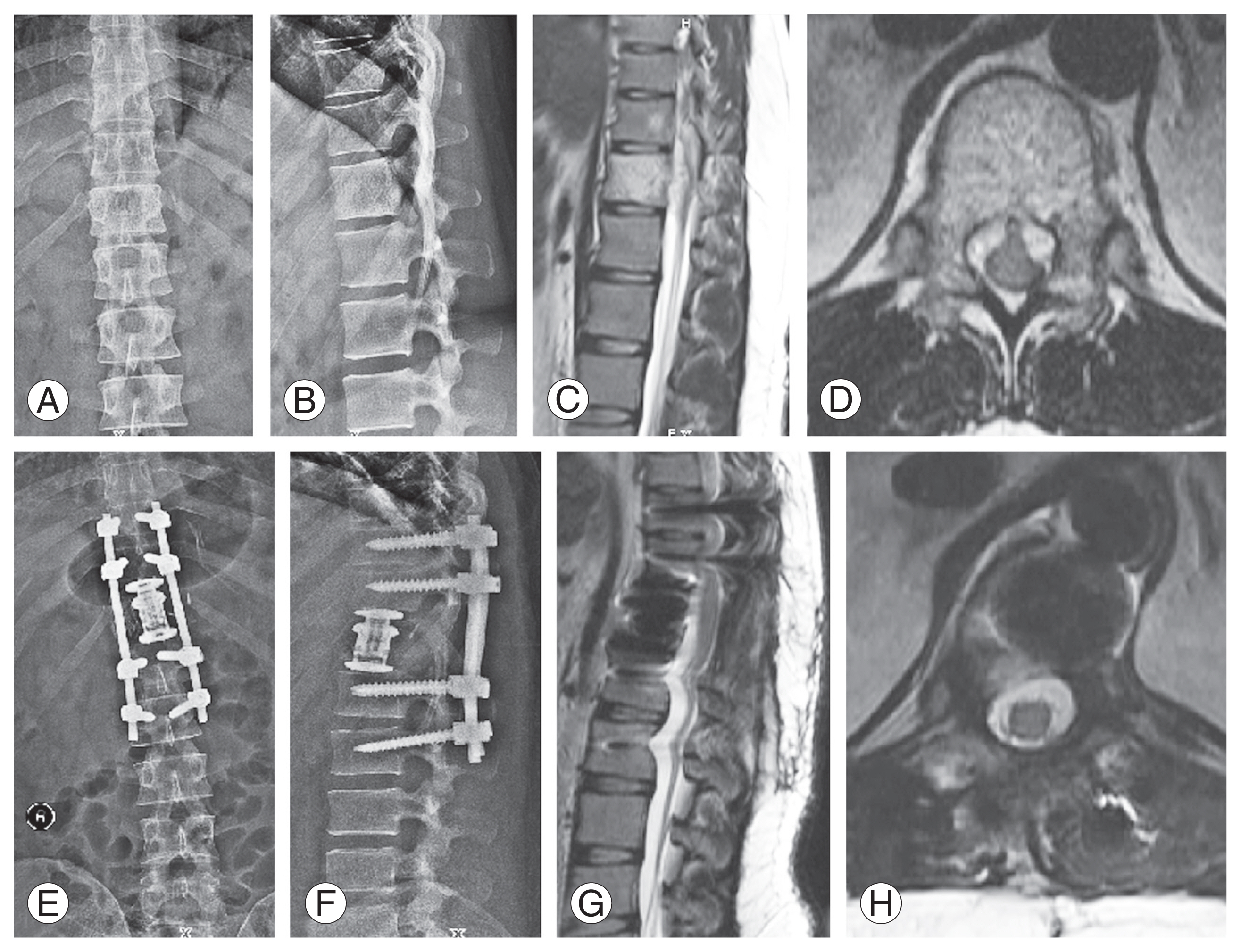
(A, B) Anteroposterior and lateral radiographs with a lesion at T12 level. (C, D) T2-weighted sagittal and axial magnetic resonance imaging (MRI) sequences showing involvement of T12 vertebra and epidural extension causing spinal cord compression. (E, F) Postoperative radiographs showing T10–L2 posterior instrumented decompression with intralesional tumor excision and cage reconstruction. (G, H) Postoperative follow-up MRI showing adequate spinal cord decompression.
Recurrence was noted in a patient who underwent vertebroplasty with instrumented decompression at the 3-year follow-up. This patient was a 14-year-old girl with T2 aggressive hemangioma classified as WBB 1–12 with a SINS score of 12 (Fig. 3). Three years after the index procedure, the patient had a recurrence. Therefore, she underwent a staged posterior–anterior surgery following preoperative embolization. In the first stage, wider decompression of the cord with extension of instrumentation was performed posteriorly, followed by T2 corpectomy with cage reconstruction via an anterior approach (Fig. 4). Postoperatively, the patient was normal without further recurrence until her last follow-up 2 years after. No recurrence was noted in the intralesional tumor resection group. The treatment details of all study patients are shown in Table 2.
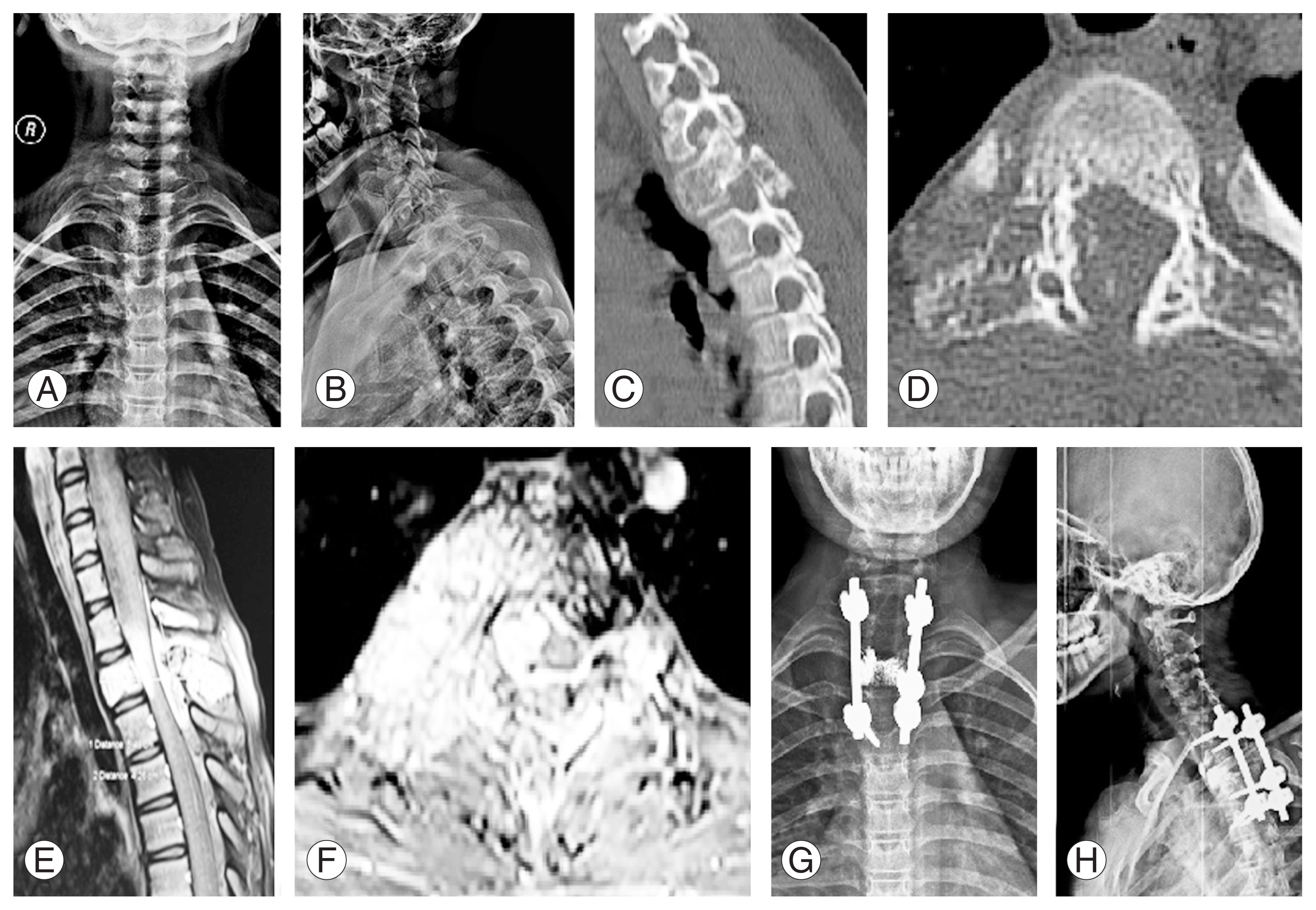
(A, B) Anteroposterior and lateral radiographs of a 14-year-old female showing a lesion at T2 level. (C, D) Computed tomography images shows a lytic lesion in sagittal and axial images involving the vertebral body, bilateral pedicles, transverse process, and articular pillar. (E, F) Post-contrast magnetic resonance imaging sequences shows epidural extension (Weinstein–Boriani–Biagini classification: layer [D/E]) and cord compression. (G, H) Postoperative radiographs after C7–T4 posterior instrumented stabilization, T2 vertebroplasty, and laminectomy.
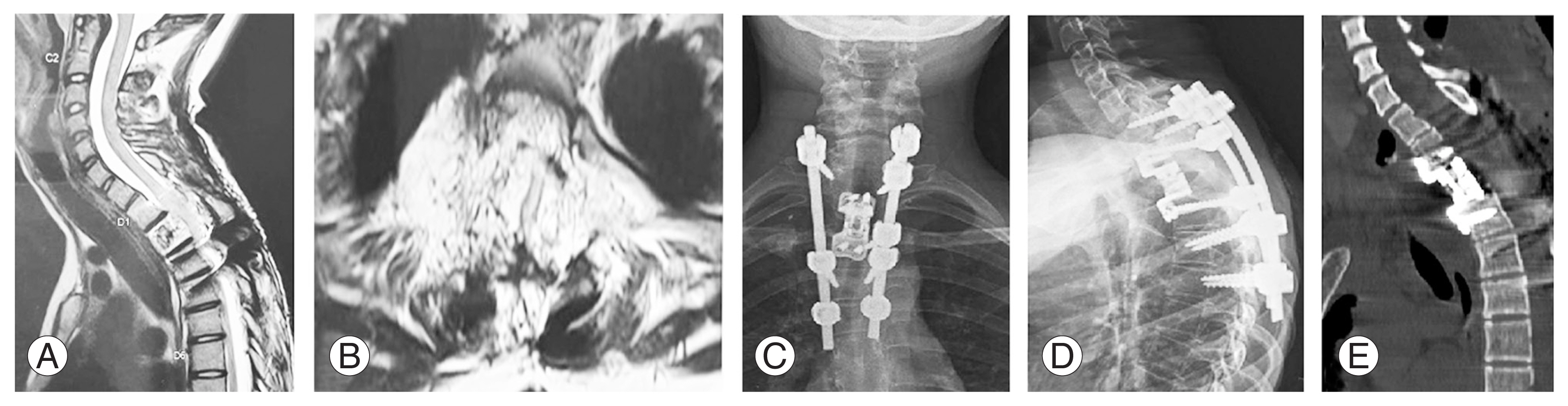
(A, B) Magnetic resonance images showing recurrence of the tumor at the T2 level at a 3-year follow-up. (C, D) Anteroposterior and lateral radiographs after corpectomy and anterior reconstruction. (E) Computed tomography showing appropriate placement of the cage.
5. Outcomes
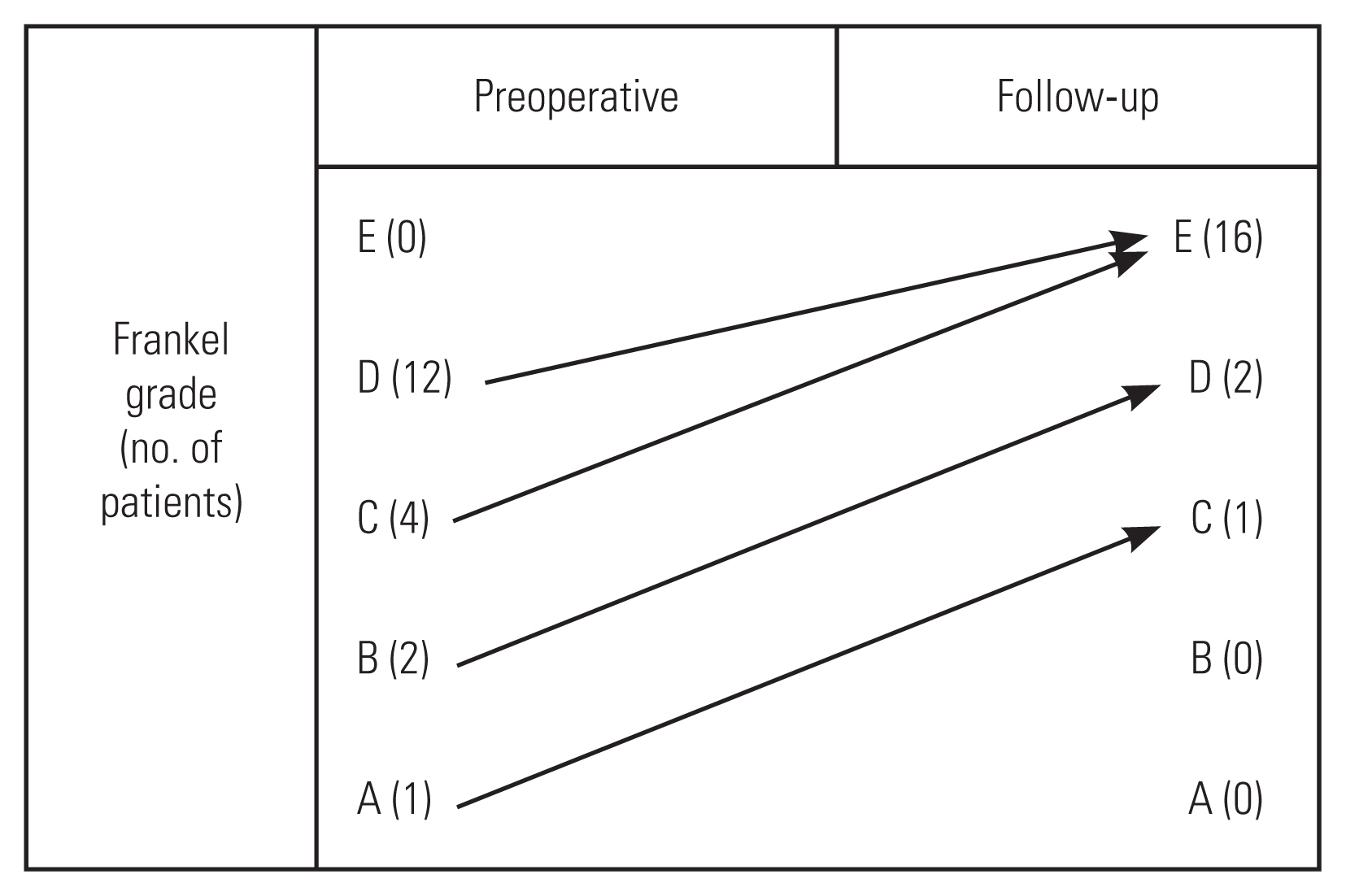
The mean follow-up duration was 55.78±25.03 months (range, 24–96 months). During follow-up, all patients had neurological improvement. One patient with Frankel A improved to Frankel C, and two patients with Frankel B recovered to Frankel D. Patients with Frankel C and D had complete recovery of neurological function (Frankel E) (Fig. 5). Patients with cauda equina symptoms also had complete neurological recovery.
Discussion
VHs are the most common benign tumors of the spine. Though they are grouped as benign tumors, they are vascular malformations (hamartomas) of capillary and venous structures [12]. The incidence of VHs is common in females with male-to-female ratios varying from 1:1.3 to 1:2.25 [4]. AVHs are a rare subset of hemangiomas (1%) that exhibit features of extra-osseous invasion, bony expansion, cortical breach, and invasion of the epidural and paravertebral space, leading to pathological fractures or neurological deficit. Approximately 45% of AVH cases present with neurological symptoms because of compressive myelopathy [23]. Cord compression in AVH can be caused by posterior cortical wall ballooning, epidural soft tissue expansion, acute hemorrhage into the epidural space, or a pathological collapse. In view of worsening neurology, early intervention is recommended aiming at neurological recovery and achieving optimal functional status.
The most common location of AVHs is the thoracic spine [24]. They are more likely to be symptomatic in this region because of the narrow vertebral canal dimensions. Then, most patients (57.1%) in this study had a lesion in the thoracic spine, followed by the thoracolumbar spine (33.3% of patients). Multiple VHs are common and have been reported. In the study by Jiang et al. [12], four of 29 patients had hemangiomas at multiple levels. However, only one of the lesions showed features of AVH; the rest were benign and inactive. In this study, 10 patients had multiple lesions, and only one patient had no symptoms. The SINS is used to assess the tumor-related instability of the spinal column. Because there are no other scoring systems for assessing the stability of the spine in benign tumors, the SINS was used in this study. The average SINS class in this study was class II with a mean score of 9.5 (range, 7–12). Though all lesions had a cortical breach, none of the lesions were unstable (range, 13–18) according to the SINS class.
Early surgical decompression is indicated in the presence of progressive neurological deficit or myelopathy and offers the best outcomes. Surgical treatment options include decompression+vertebroplasty, intralesional spondylectomy, en bloc spondylectomy with stabilization, or reconstruction of the vertebral defect. Because of the inherent vascularity of VHs, preoperative embolization is favorable for reducing blood loss during surgery. A recent meta-analysis by Robinson et al. [25] concluded that the preoperative embolization group bled less (980±683 mL) than the non-embolized group (1,629±946 mL). Preoperative embolization was performed in all patients in this study with a maximum blood loss of 1,700 mL. None of the patients in this study experienced a massive hemorrhage.
Posterior decompression had been an accepted technique in patients with AVH. Dobran et al. [26] suggested that posterior laminectomy can be performed in patients with AVH with cord compression because of the epidural soft tissue component. In their study, Urrutia et al. [27] reported complete neurological recovery following instrumented decompression. In this study, decompressive laminectomy with stabilization was performed in three patients who presented with nonprogressive mild neurological deficits. Furthermore, all three patients showed neurological recovery at the follow-up.
The role of vertebroplasty in VHs has been well documented in the literature. Deramond et al. [28] first performed vertebroplasty in the AVH of atlas. In aggressive lesions with cortical destruction, performing vertebroplasty is beneficial as it helps prevent a pathological collapse and provides anterior column support. Studies have also supported the fact that injected cement decreases the vascularity of the lesion, decreasing blood loss during decompression, and aids in the shrinkage of the tumor. Jiang et al. [12] noticed a decreased blood loss in patients who underwent vertebroplasty and decompression compared with patients who underwent decompression without vertebroplasty (1,093 mL versus 1,900 mL), and similar results were observed in this study (561 mL versus 663 mL). In our patients, after instrumented laminectomy, cement was injected using the transpedicular approach. This reduced bleeding from the lesion as a wider decompression was then performed. In a study by Shamhoot et al. [29], vertebroplasty with decompression showed promising outcomes, and the authors concluded it to be an effective procedure without the risk of recurrence. In our series of nine patients, all nine patients underwent vertebroplasty with instrumented decompression, and only one case of recurrence was noted at the 3-year follow-up. The patient had extensive involvement of all zones according to the WBB classification (zones: 1–12; layer: D/E). Then, she underwent a staged posterior–anterior surgery, and total en bloc corpectomy with cage reconstruction was performed (Fig. 4).
En bloc spondylectomy provides wide surgical margins but is associated with significant perioperative morbidity because of excessive blood loss. Acosta et al. [23] reported a mean blood loss of 2.1 L in 10 patients who underwent en bloc spondylectomy. Similarly, Ji et al. [30] in a recent study of 23 patients with Enneking stage 3 AVH reported a maximum blood loss of 2.8 L (embolization group) and 4 L (non-embolization group) during total en bloc spondylectomy. Hence, several authors suggest using less invasive approaches, such as intralesional tumor resection, to provide satisfactory results with relatively lesser blood loss. In this study, intralesional tumor resection, decompression, and stabilization were performed in nine patients with a maximum blood loss of 1.7 L. Goldstein et al. [31] in a multicenter study reported that in 33 patients with VH with epidural extension, intralesional resection was associated with minimal recurrence (3%) and an excellent survival rate. Furthermore, we believe that intralesional tumor resection can obliterate the vascular channels and significantly reduce the risk of recurrence. In this study, no recurrences were observed among the patients who underwent intralesional resection. Even in patients with extensive involvement of all zones (1–12) according to the WBB classification, intralesional excision resulted in satisfactory neurological outcomes without recurrence.
The limitations of this study include the retrospective study design with a relatively shorter follow-up period; furthermore, heterogeneous treatment modalities with few patients in each group make comparisons difficult. Patient outcomes based on health-related quality of life were not assessed in this study. Nevertheless, considering the rarity of AVH, there are very few studies in the literature with a larger sample size.
Conclusions
In AVH with neurological deficits, less extensive approaches, such as posterior decompression with vertebroplasty and intralesional tumor resection with anterior reconstruction, offer good clinical and neurological outcomes with less recurrence, and en bloc spondylectomy may rarely be needed.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception or design of the work; or the acquisition, analysis, or interpretation of data for the work: all authors; drafting the work or revising it critically for important intellectual content: all authors; final approval of the version to be published: all authors; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved: all authors.