Multicenter Prospective Study of Lateral Lumbar Interbody Fusions Using Bioactive Porous Titanium Spacers without Bone Grafts
Article information
Abstract
Study Design
Prospective multicenter clinical study.
Purpose
To evaluate the efficacy of bioactive surface treatment for porous titanium spacers without bone graft for lateral lumbar interbody fusion (LLIF) through clinical and radiological examinations.
Overview of Literature
LLIF is a minimally invasive spinal fusion procedure. To achieve bony union, a substantial volume of grafted bone is typically packed into the cage; however, this is related to donor site morbidities—one of the disadvantages of LLIF.
Methods
For this prospective multicenter study, 40 patients were followed up through radiologic and clinical examinations for at least 1 year postoperatively. All surgical procedures were either single- or double-level LLIF using bioactive porous titanium spacers without bone grafts.
Results
Four patients were excluded from the study owing to aggravation from other comorbidities. Another 36 patients, including 26 and 10 with single- and double-level LLIFs, respectively, participated in the follow-up. The mean age at the time of surgery was 63.7 years. The mean operating time was 50.5 minutes per level. The mean estimated intraoperative blood loss was 11.6 mL per level. Clinical scores improved in all cases and were maintained throughout the follow-up period. The intervertebral bony union rates were 67.4% and 84.8% at 6 and 12 months, respectively. Endplate cyst signs were observed in 13.0% and 8.7% of patients at 6 and 12 months, respectively. Fused segmental angles were maintained throughout the follow-up period, indicating no cage subsidence.
Conclusions
Single- and double-level LLIFs using bioactive porous titanium spacers without bone grafts were found to be minimally invasive, resulting in clinical and imaging results comparable with conventional procedures. Therefore, this type of implant may be an option for minimally invasive spinal fusion surgery.
Introduction
Lateral lumbar interbody fusion (LLIF) is a minimally invasive spinal fusion procedure [1–3]. The following are the two types of LLIF: extreme lateral interbody fusion (XLIF) and oblique lateral interbody fusion (OLIF). Although XLIF and OLIF have their advantages and disadvantages, they have comparable complication rates in large complication studies [4], and the choice between the two techniques is generally based on surgeon preference. The advantages of LLIF include high initial stability with ligamentotaxis, high corrective strength for coronal and sagittal planes, indirect neural decompression, and high bony union rate owing to the large footprint of the cage. To achieve bony union, a substantial volume of grafted bone is typically packed into the cage; however, this is related to donor site morbidities—one of the disadvantages of LLIF. The gold standard graft material is autologous bone; however, there are complications associated with harvesting a large amount of bone from the ilium, including problems with the quality and quantity of the grafted bone [5]. Allogeneic bones, artificial bones, and bone morphogenetic proteins (BMPs) instead of autologous bones are used to decrease donor site morbidities; however, they also have drawbacks. For example, allogeneic bone is associated with viral infections; artificial bone with low mechanical strength and low bone fusion rates; and BMPs with high costs, specific complications, including soft tissue swelling, bone resorption, and retrograde ejaculation and are not approved for use in all countries [6–8]. Several types of cage materials are available for clinical use, including polyetheretherketone (PEEK), titanium-coated PEEK, and titanium metal and its alloys. Although titanium metal and its alloys are biocompatible materials, they do not bond with living bone. There are several surface treatments to accelerate the bonding of titanium metal and its alloys to the bone, such as plasma spray coating, hydroxyapatite coating, and alkali and heat treatment [9,10]. Alkali and heat treatment has already been applied in cementless total hip arthroplasty (THA), and excellent clinical results have been reported in Japan [11]. The treatment is relatively simple and can be applied to surfaces with complex morphologies, such as porous bodies. By making the titanium metal a porous body, it is possible to make a material with higher biocompatibility while maintaining its strength [12]. Animal experiments have revealed that bioactive porous titanium is osteoconductive and osteoinductive without the additional use of osteogenic cells or agents, such as BMPs [13,14]. Clinical trials of porous bioactive titanium spacers for transforaminal lumbar interbody fusion have shown excellent bone bonding ability and clinical results [15]. Given these encouraging results, a bioactive porous titanium spacer was developed for LLIF and approved as an LLIF spacer without bone grafts by the Pharmaceuticals and Medical Devices Agency in Japan in 2018.
In the current prospective multicenter study, clinical and radiological results from patients with bioactive porous titanium spacers for LLIF without bone grafts were evaluated.
Materials and Methods
1. Surgical procedure
In this study, XLIF with neuromonitoring was performed in all cases. Intraoperative neuromonitoring and supplemental posterior pedicle screw fixation were performed in all cases. Additional autologous bone grafts, synthetic or allogenic bone grafts, and osteogenic cells or agents, including BMPs, were not used in any of the cases. Neural decompression from the posterior region was added at the operator’s discretion.
2. Clinical assessments
A multicenter study was conducted at three institutions. All surgical procedures were performed by two senior authors (S.F. and K.I.). Forty patients were prospectively enrolled. Radiological and clinical data were collected for at least 1 year postoperatively. Study approval was obtained from the three institutional ethics committees before the study began (R1955). Written informed consents were obtained from the patients.
The following were the inclusion criteria: (1) persistent back and/or leg pain (LP) unresponsive to conservative treatment for at least 3 months, (2) indications for lateral interbody fusion, (3) one or two contiguous levels between L1 and L5, and (4) agreement to return for post-treatment examinations according to the follow-up protocol. The following were the exclusion criteria: (1) the presence of a tumor and/or infectious disease and (2) patients who did not follow-up/did not meet the criteria for LLIF. Surgeries were performed between August 2018 and June 2019. The surgical procedure was a single- or double-level LLIF. Intra- and perioperative complications were recorded during follow-up. Clinical results were assessed using the Japanese Orthopedic Association (JOA) score (full score=29), Oswestry Disability Index (ODI) score (%), and self-assessed 100-mm Visual Analog Scale (VAS) (0 mm=no pain, 100 mm=worst pain imaginable) for both low back pain (LBP) and LP preoperatively and at 1, 3, 6, and 12 months postoperatively.
3. Radiological assessments
Radiological assessments were performed using dynamic X-ray and computed tomography (CT) preoperatively and at 6 and 12 months postoperatively. A motion of more than 3° during flexion-extension was considered nonunion. To evaluate bony union, coronal and sagittal multi-detector (MD) CT reconstruction views were assessed. Bony union was considered complete when there was osseous continuity between the bony endplate and the implant on both the coronal and sagittal MDCT images. Radiolucency >50% over the anteroposterior distance of the interface between the endplates and implants was defined as nonunion. Successful bony union was recorded when the abovementioned radiological parameter assessments were complete [15]. The presence or absence of cyst formation on the endplate of the vertebral body in contact with the spacer was also evaluated [16]. To evaluate the cage subsidence, fused segment angles (FSAs) were assessed preoperatively, immediately postoperatively, and at 1, 3, 6, and 12 months postoperatively. Bony union was evaluated for each case and each intervertebral space. To determine the union status, all imaging studies were reviewed by the senior author (S.F.). To evaluate the interobserver reliability of the radiological assessments, another independent orthopedic surgeon (T.S.) blindly and independently reviewed consecutive 20 cases, and these results were compared with those obtained by the senior author. Interobserver reliability was statistically evaluated for the union status.
4. Statistical analysis
All statistical analyses were performed using JMP Pro ver. 15 (SAS Institute Inc., Cary, NC, USA) using paired t-tests, and p-values <0.05 were considered statistically significant. The kappa coefficient was calculated to compare the interobserver reliability of the radiological evaluation.
5. Bioactive porous titanium spacer
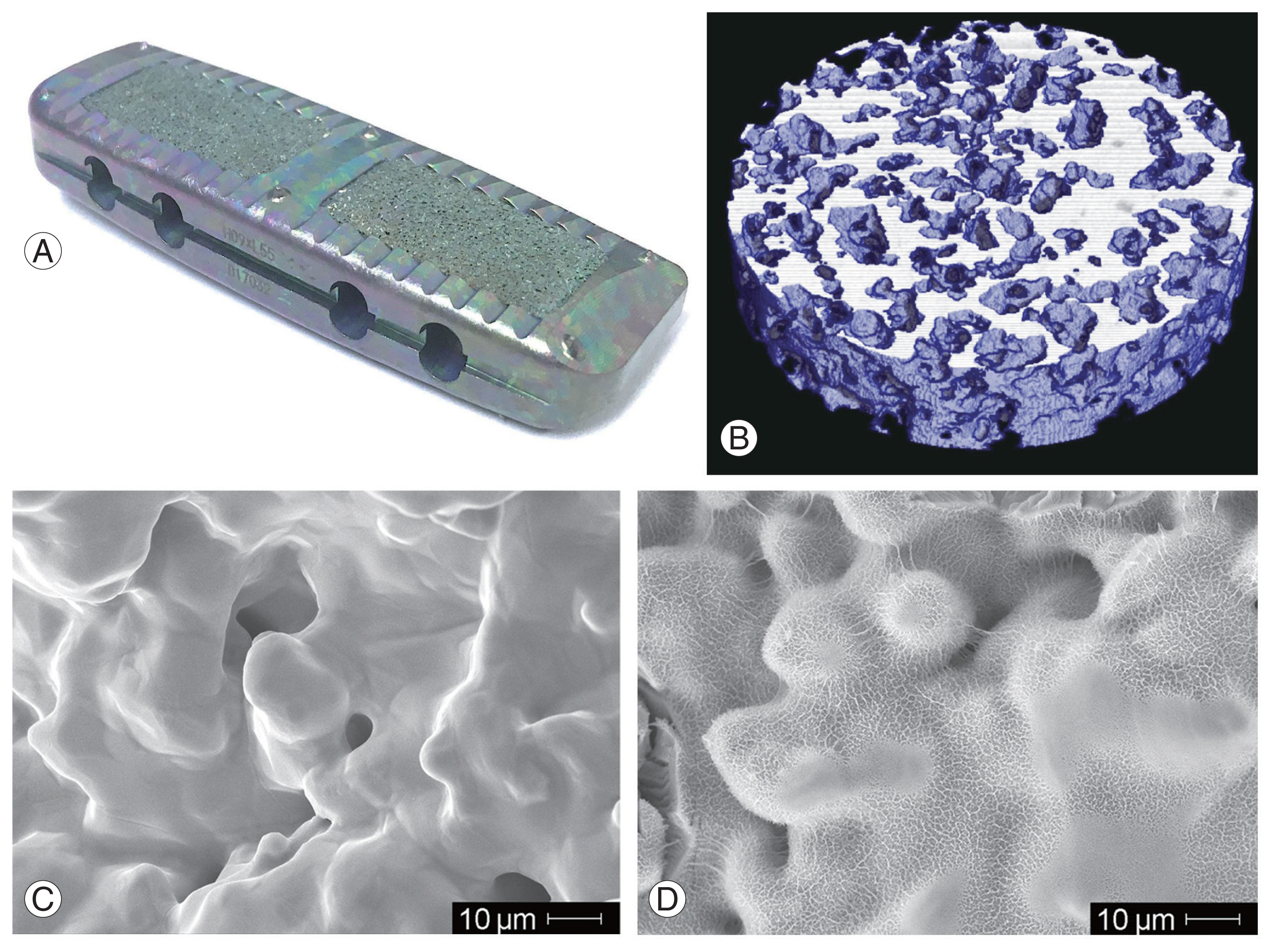
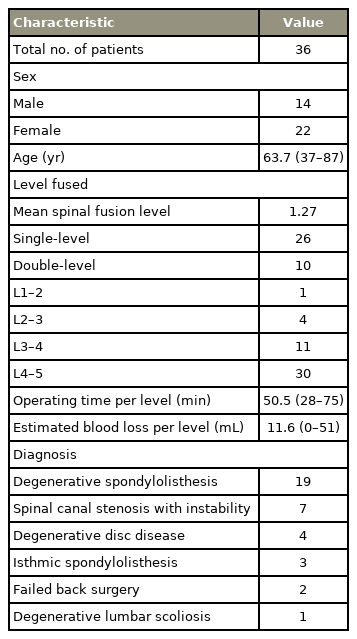
A bioactive porous titanium spacer (X-TAL; Nuvasive Japan, Tokyo, Japan and Osaka Yakin Kogyo Co. Ltd., Osaka, Japan) was built into a porous body and outer frame (Fig. 1A). A porous titanium body was manufactured from a mixture of commercially pure titanium powder with a particle size of <45 μm (Osaka Titanium Technologies Co. Ltd., Osaka, Japan) and ammonium hydrogen carbonate as spacer particles (Fig. 1B). Sintering was performed at 1,400°C for 2 hours in argon gas. More than 99% of the porous structures were interconnected through the channels. The average porosity was 60%, and the average pore size was 250 μm (Fig. 1C). The compressive strength and Young’s modulus of the porous body were 53.0 MPa and 4.2 GPa, respectively. The implant was treated chemically and thermally to provide a bioactive surface (Fig. 1D). Briefly, the treatment conditions were immersed in a 5-M aqueous sodium hydroxide solution at 60°C for 24 hours, ultrapure water at 40°C for 24 hours, and subsequently heat-treated at 600°C for 1 hour. After the treatment, a thin (approximately 1 μm) densified dehydrated amorphous sodium titanate bioactive layer was formed on the titanium surface. Bone-to-titanium substrate bonding occurred via the thin bioactive layer [9].
Results
Four patients were excluded from the study during the follow-up period due to worsening of comorbidities not related to the surgery. The first case was a 76-year-old man. After 6 months of follow-up, pancreatic cancer was detected, and he discontinued follow-up after the cancer rapidly worsened. The second case was an 80-year-old man. After 3 months of follow-up, he developed paralysis due to multiple myeloma of the thoracic spine and dropped out of the follow-up. The third case was a 78-year-old man. He was lost to follow-up after 1 month as he developed paralysis due to spinal cord infarction. The fourth case was a 74-year-old man. Follow-up was possible until 6 months postoperatively; however, he dropped out of follow-up due to the worsening of Parkinson’s disease. Another 36 patients (14 male patients, 22 female patients), comprising 26 and 10 who underwent single- and double-level LLIFs, respectively, participated in the follow-up assessment for at least 1 year. For posterior fixation, percutaneous pedicle screw fixation (PPS) was performed in 33 cases, and open fixation was performed in three cases. Neural decompression was noted in five of the 36 cases. Bone grafting was not performed for any of the patients. A total of 46 levels from 36 patients with a mean age of 63.7 years (range, 37–87 years) at the time of surgery were assessed through clinical and radiological examinations. The mean spinal fusion level was 1.27. The mean operating time was 50.5 minutes (range, 28–75 minutes) per level, and the mean estimated intraoperative blood loss was 11.6 mL (range, 0–51 mL) per level. The preoperative pathologies included degenerative spondylolisthesis, spinal canal stenosis with instability, degenerative disc disease, isthmic spondylolisthesis, failed back surgery, and degenerative lumbar scoliosis in 19, seven, four, three, two, and one case, respectively (Table 1).
1. Clinical assessments
For the 36 patients, no complications were reported during the follow-up period. The mean preoperative JOA score was 15.0 points, which improved to 24.0 points 1 month postoperatively. The mean JOA score remained stable throughout the follow-up period. The mean preoperative ODI score was 43.1%, which improved to 19.3% 1 month postoperatively. The mean ODI score remained stable throughout the follow-up period. Both the JOA and ODI scores were significantly improved compared with preoperative scores at each follow-up (p<0.001 at 1, 3, 6, and 12 months). The mean preoperative LBP VAS score was 50.4 mm, which improved to 13.8 mm 1 month postoperatively. The mean LBP VAS score remained stable throughout the follow-up period. The mean preoperative LP VAS score was 64.9 mm, which improved to 11.1 mm 1 month postoperatively. The mean LP VAS score remained stable throughout the follow-up period. Both VAS measures were significantly improved compared with preoperative scores at each follow-up (p<0.001 at 1, 3, 6, and 12 months) (Table 2).
2. Radiological assessments
At 6 and 12 months, the bony union rate for each case was 66.7% and 80.1%, respectively, and the intervertebral union rates were 67.4% and 84.8%, respectively. The preoperative mean FSA was 10.9°, which increased to 17.0° postoperatively. The mean FSA remained stable throughout the follow-up period. The serial changes in the postoperative FSA were not statistically significant at any time point (1 month versus 3 months, p=0.776; 3 months versus 6 months, p=0.891; and 6 months versus 12 months, p=0.323). Positive cyst signs were observed in six levels (13.0%) at 6 months, which decreased to four levels (8.7%) at 12 months (Table 3). The kappa coefficient was 0.842 for the interobserver reliability between the two independent observers for the union status, indicating high reliability for the two independent observations.
3. Case illustration
A 76-year-old woman with degenerative spondylolisthesis at L4–5. Her preoperative JOA and ODI scores were 13 and 22 points, respectively. The VAS score was 50 mm for LBP and 50 mm for LP. X-ray images revealed degenerative spondylolisthesis at the L4–5 level with instability. Preoperative magnetic resonance imaging (MRI) revealed severe spinal canal stenosis at the L4–5 level (Fig. 2A, B). Single-level LLIF using a bioactive porous titanium spacer and supplemental PPS was performed. Immediate postoperative MRI demonstrated indirect neural decompression (Fig. 2C, D), and coronal CT imaging demonstrated a radiolucent line between the implant and vertebral endplate (Fig. 3A). The radiolucent line decreased 6 months postoperatively and disappeared at 12 months, demonstrating complete bony union (Fig. 3B, C). The state of the bony union was confirmed in the metal artifact reduction reconstruction image at 12 months (Fig. 3D). At 12 months postoperatively, the patient’s JOA and ODI scores recovered to 28 and 10 points, respectively. The VAS score decreased to 0 mm for LBP and 25 mm for LP.
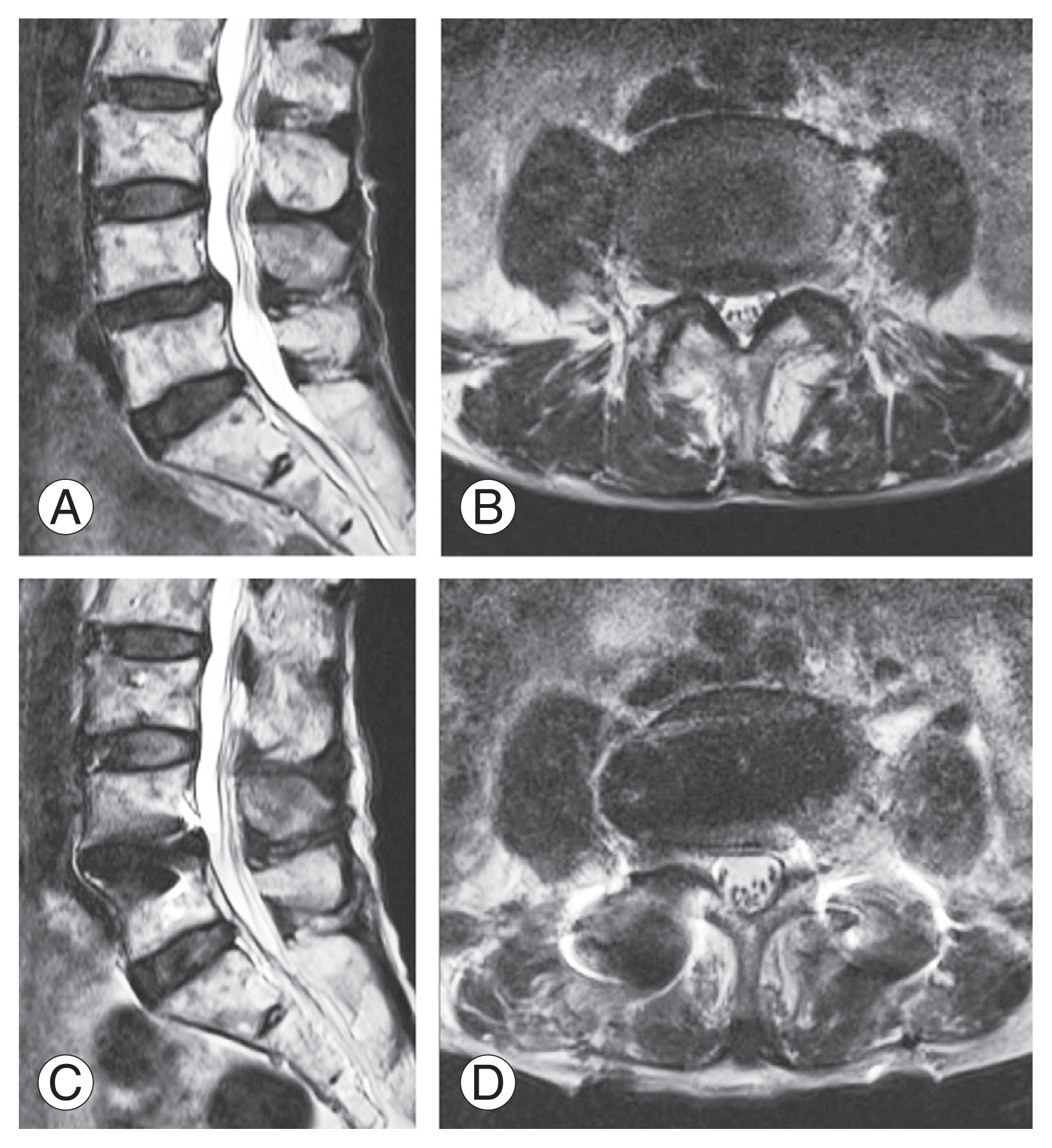
(A) Preoperative sagittal magnetic resonance (MR) image. (B) Preoperative axial MR image. (C) Postoperative sagittal MR image. (D) Postoperative axial MR image.
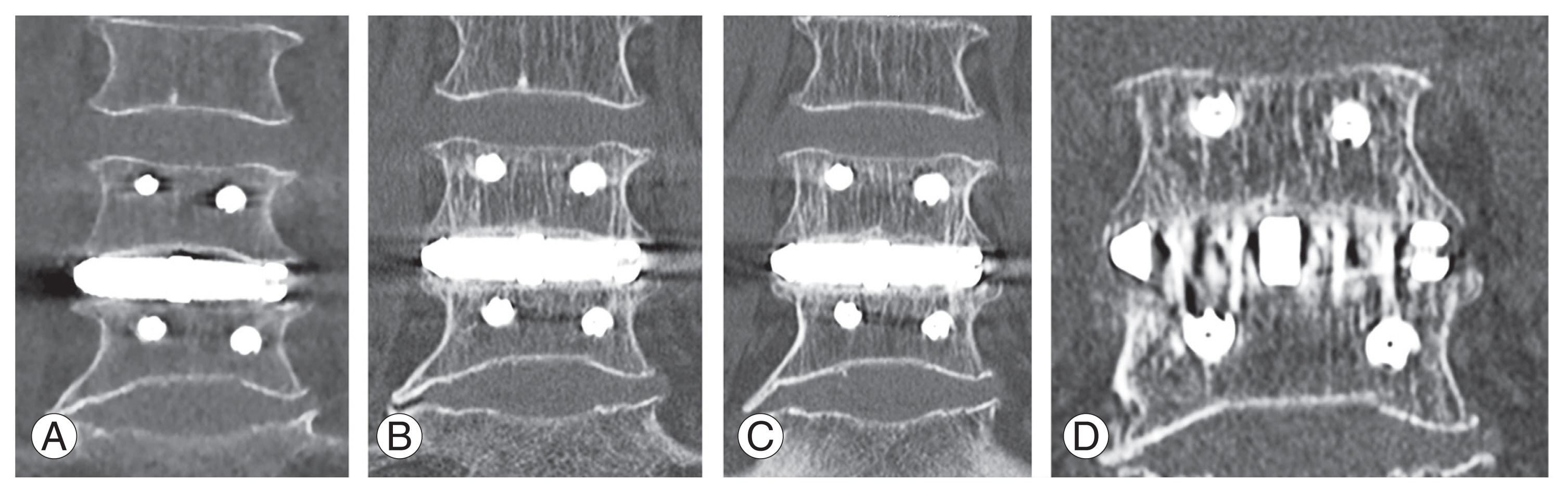
(A) Coronal computed tomography (CT) image immediately after surgery showing a clear gap between the implant and vertebral endplate. (B) Coronal CT image 6 months after surgery showing a decrease in the gap. (C) Coronal CT image 12 months after surgery showing the gap filled with new bone. (D) Metal artifact reduction reconstruction image 12 months after surgery showing trabecular integration between bone to implant.
Discussion
A bioactive porous titanium spacer for LLIF was clinically and radiologically evaluated in this prospective multicenter study. Although this implant did not use autologous bone grafting, the bony union rate was as high as that of conventional LLIF with autogenous bone grafting [17] and higher than the rate using artificial bone [18]. Several materials can be used to fill the cage, including autogenous bone, BMP, demineralized bone matrix, and artificial bone. All these materials have their advantages and disadvantages. The following are the advantages of the porous titanium cage: does not require autologous bone harvesting, does not require filling the cage since it is already filled, has no risk of falling out of the cage during insertion, has stable osteoconductive ability at all times, and has the same price as a normal cage.
In this study, early bone bonding of the bioactive porous titanium spacers was observed. The mechanism of bony union through bioactive porous titanium spacers may be different from that of conventional hollow cages, which require osseous continuity through the cage to achieve complete bony union. Therefore, in cases of wide disc space, sufficient internal bone growth is delayed. Conversely, bioactive porous titanium spacers achieve bony union on the surface, which is related to early bone bonding.
A mismatch between the vertebral endplate shape and the spacer surface shape was occasionally observed on CT assessments. The shape of the vertebral endplate was variable and depended on the patient. In the case of osteoporosis, the shape of the vertebral endplate may be concave, relating to the morphological mismatch between the cage and the vertebral endplate. Typically, a mismatch affects cage subsidence and nonunion [19]. However, in this study, the gap between the vertebral body and the implant was filled with a new bone. This phenomenon was observed in cementless THA using an alkali and heat treated implant, which indicated high osteoconductivity of the implant [11].
The presence or absence of grafted bone trabeculation inside the cage is generally used to determine bony union in interbody fusion; however, due to the metal artifacts, observing the inside of a porous titanium implant is very difficult. Therefore, to assess bony union, we evaluated the changes in radiolucent lines on the bone and implant surfaces using CT and the presence or absence of intervertebral mobility using functional radiography. The presence or absence of cyst formation around the cage in the early postoperative period is a factor in predicting bony union, and de novo cyst formation and cyst enlargement are image findings indicating nonunion [15]. Therefore, the presence or absence of cyst formation was evaluated in this study. Tanida et al. [20] reported that the bony union rate increased over time with a decrease in the cyst sign; in our case, the bony union rate may further increase with the decrease in the cyst sign, and we believe that long-term follow-up is required. Although it is impossible to conclude the results since it was not performed in all cases, the method of metal artifact reduction in the reconstructing image shown in Fig. 2D has been shown to be effective in evaluating the interface between the metal implant and the bone [21].
This study had several limitations. First, the surgical indications were limited to the pathologies for single- or double-level fusion. Since this was the first study of bioactive porous titanium spacers without bone grafts for LLIF, to evaluate the isolated effect of the implant, it was necessary to limit the surgical procedure as much as possible. Second, the bone mineral density was not evaluated. The mean age of the patients in this study was 63.7 years, and it seems that there were several cases of osteoporosis included; however, appropriate clinical results were achieved. However, the bony union and implant subsidence in patients with osteoporosis need to be evaluated. Third, the follow-up period was only 1 year postoperatively. Long-term follow-up studies are needed to evaluate the true effects of the implant. Finally, owing to the lack of a control group, the results of this study do not allow us to draw strong conclusions. Further comparative studies are needed to evaluate the advantages and disadvantages of implants.
Conclusions
A bioactive porous titanium spacer for LLIF was evaluated through clinical and radiological examinations in this prospective multicenter study. Although the implant did not use autologous bone grafting, the bony union rate was comparable with that of a conventional hollow cage with an autologous bone graft. This type of implant may be an option for minimally invasive spinal fusion surgery.
Acknowledgments
Kyoto University Hospital, Kyoto City Hospital, and International University of Health and Welfare Mita Hospital received financial support from Osaka Yakin Kogyo Co. Ltd. for the cost of post-marketing surveillance.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: SF, MT; data acquisition: SF, MT, KI, HF, NI, BO, TS; analysis of data: SF, TS. drafting of the manuscript: SF; critical revision: MT, KI, HF, NI, BO, TS; obtaining funding: none; supervision: TN, SM; and all authors read and approved the final manuscript.