Cervical Radiculopathy: Focus on Factors for Better Surgical Outcomes and Operative Techniques
Article information
Abstract
For patients with cervical radiculopathy, most studies have recommended conservative treatment as the first-line treatment; however, when conventional treatment fails, surgery is considered. A better understanding of the prognosis of cervical radiculopathy is essential to provide accurate information to the patients. If the patients complain of persistent and recurrent arm pain/numbness not respond to conservative treatment, or exhibit neurologic deficits, surgery is performed using anterior or posterior approaches. Anterior cervical discectomy and fusion (ACDF) has historically been widely used and has proven to be safe and effective. To improve surgical outcomes of ACDF surgery, many studies have been conducted on types of spacers, size/height/position of cages, anterior plating, patients’ factors, surgical techniques, and so forth. Cervical disc replacement (CDR) is designed to reduce the incidence of adjacent segment disease during long-term follow-up by maintaining cervical spine motion postoperatively. Many studies on excellent indications for the CDR, proper type/size/shape/height of the implants, and surgical techniques were performed. Posterior cervical foraminotomy is a safe and effective surgical option to avoid complications associated with anterior approach and fusion surgery. Most recent literature demonstrated that all three surgical techniques for patients with cervical radiculopathy have clear advantages and disadvantages and reveal satisfactory surgical outcomes under a proper selection of patients and application of appropriate surgical methods. For this, it is important to fully understand the factors for better surgical outcomes and to adequately practice the operative techniques for patients with cervical radiculopathy.
Introduction
Patients with cervical radiculopathy present neck and arm pain, sensory loss, motor dysfunction, and reflex changes according to dermatomal distribution. The natural history of cervical radiculopathy was generally favorable, and most studies have recommended conservative treatment as the first-line treatment [1,2]. Meanwhile, the prognosis was poor when symptoms had a longer duration, absence of paresthesia, higher neck pain intensity at baseline, higher baseline disability, and a lower active range of motion (ROM) on the symptomatic side [3].
Representative surgical procedures are anterior cervical discectomy and fusion (ACDF), cervical disc replacement (CDR), and posterior foraminotomy (PCF). Each procedure has strengths and weaknesses and is performed according to slightly different indications and contraindications. The surgery should be considered when conservative treatment fails or neurologic deficits progress.
A thorough understanding of the prognostic factors for better surgical outcomes of cervical radiculopathy is vital to improve surgical results and provide accurate information to patients. Although numerous studies for patients with cervical radiculopathy have been conducted, there is still little data for evaluating factors for better surgical outcomes. This study aims to evaluate the factors for better surgical outcomes and to learn safe and effective surgical techniques for patients with cervical radiculopathy.
Anterior Cervical Discectomy and Fusion
The ideal surgical treatment of cervical radiculopathy remains controversial. ACDF is widely used as the “gold standard.” In single-level unilateral cervical radiculopathy, a randomized controlled trial by Engquist et al. [4] demonstrated rapid improvement in neck disability, health status, neck pain, and arm pain in patients who received ACDF surgery. These scores indicated a gradual improvement from 2 to 5–8 years, although no further improvement was observed in the group that received nonsurgical treatment [4,5].
1. Indication and contraindication
ACDF is the most common procedure for symptomatic cervical radiculopathy and is not compromised by conservative treatment. Indications of ACDF include persistent and recurrent arm pain/numbness that does not respond to conventional treatment, neurologic deficits (especially definite weakness), confirmatory axial imaging studies consistent with the patient’s clinical findings, and traumatic instability. Relative contraindications of ACDF include vertebral artery anomalies (transverse foramen medial migration), retro-vertebral body compressive pathology (requiring corpectomy), and posterior neural compression [6].
2. Factors for better surgical outcomes
Iliac crest bone grafting has been considered the gold standard for intervertebral body implants. Recently, many studies have been conducted to identify alternatives to autogenous bone grafts, including allografts and synthetic and factor/cell-based grafts and cages.
1) Allo-bone grafts
Yue et al. [7] investigated the duration of ACDF maintenance using allografts and observed improvement in symptoms of neck pain and radiating pain in >95% of patients who received allograft and plating during a follow-up period of 5–11 years. A study has been conducted comparing intervertebral cages and structural allografts used in ACDF. Pirkle et al. [8] performed a retrospective analysis of 6,130 patients who underwent ACDF and investigated 2,067 patients with cage implants and 4,063 patients with allografts. When comparing the non-union rates of these two groups, the cage group (5.32%) had significantly higher non-union rates than the allograft group (1.97%) [8]. In terms of long-term prognosis, using allografts in ACDF surgery seems favorable.
2) Cage implants
Many studies have compared cage materials, polyetheretherketone (PEEK), titanium (Ti), carbon fiber-reinforced polymers, and polymethyl methacrylate. PEEK and Ti cages have relatively superior clinical outcomes, higher fusion rates, and lower subsidence rates than others. No significant differences in the fusion rates were observed between Ti and PEEK [9,10]. Meanwhile, an absorbable cage using polylactic acid–polyglycolic acid polymers theoretically has advantages, such as immediate postoperative stability and gradual disintegration to aid bone formation and solid arthrodesis. Still, its effectiveness has not been fully demonstrated [11].
PEEK has a radiolucent nature and an elastic modulus similar to the bone, and many surgeons use PEEK plastic cages during ACDF. It has a high fusion rate, low subsidence, and stability provided by the cage, and it facilitates radiographic outcomes [12]. A long-term clinical study has revealed that PEEK has some limitations, such as endplate erosion. Recent cage designs have attempted to promote early osteointegration to achieve postoperative stability and reduce complication rates. Ti could modify the surface roughness of the cage. Hence, a Ti/PEEK combined cage was created, which has both the good bioactivity of Ti and the elastic modulus of PEEK [13,14]. However, the efficacy of Ti/PEEK combined cages remains controversial, warranting long-term and extensive clinical studies.
Recently, porous three-dimensional (3D) printed Ti cages (3DTC) have been considered an option for interbody implants. 3DTC has advantages over PEEK cages in a highly porous architecture mimicking cancellous bone, which promotes bone ingrowth. Additionally, the relatively wide range of cage sizes compared to those of standard allo-bone grafts may lead to better axial load sharing and a low subsidence rate. Comparing patient groups using 3D printed cages and allografts in ACDF, 3DTC yielded similar clinical results and fusion rates to allografts but lower subsidence rates at all times [15].
3) Spacer location
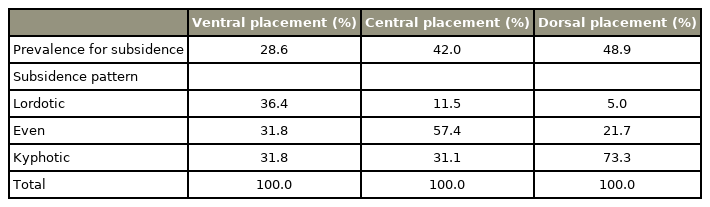
When inserting a cage for ACDF, proper size, proper location, preservation of the anterior cortical bone, and proper endplate preparation are effective in preventing subsidence and secondary kyphotic deformities [16]. In ACDF surgery, implant subsidence is crucial in determining the prognosis. In a study, surgery was performed using single-level ACDF with allografts and plates, and a retrospective study on the occurrence of subsidence was conducted. They were divided into two groups based on the allograft position: an anterior group with the allograft within 2 mm of the posterior margin of the plate and a center group with the allograft at >2 mm. The subsidence rate was 8/73 (11%) in the anterior group and 7/19 (37%) in the center group, thus indicating that the subsidence rate was lower when the allograft position was anterior [17].
Similar results were obtained for the ACDF using cages. Mende et al. [18] classified the location of the PEEK cage in the sagittal plane as ventral (anterior), central, or dorsal (posterior). Considering that all used the same rectangular PEEK cage devices, the subsidence rate decreased when placed more ventrally. The rectangular cage should be placed ventrally, although the cage was often located in a different place during surgery; only 13% of the cages were generally located in the ideal position [18] (Table 1). Barsa and Suchomel [16] and Yamagata et al. [19] also described that the subsidence rate increases as the cage is positioned away from the ventral border of the vertebra.
4) Spacer size and height
The cage/endplate ratio (=cage long length/endplate length in the sagittal plane) was confirmed to affect subsidence after ACDF. When the cage size was ≥65% of the endplate diameter measured in the sagittal plane, the subsidence rate decreased rapidly after ACDF. Therefore, when determining the cage size, a cage with a cage/endplate ratio of ≥65% can be considered to lower the subsidence rate caused by axial compression force [18].
Studies analyzing cage height and subsidence risk are limited. In one paper, the subsidence rate was significantly higher when using 6.5 or 7.5 mm than when using 4.5 mm or 5.5 mm [19]. Thus, a larger cage height is associated with subsidence because a larger height may result in larger stress on the vertebral endplate. Truumees et al. [20] have demonstrated that distraction increases can contribute to graft failure, subsidence, and non-union by increasing the pressure load on the endplate. An et al. [21] conducted a cadaveric study. When the baseline disc heights were 3.5–6.0 mm, 2–3 mm of distraction from patients’ original disc height was desirable for obtaining maximal change in foraminal size [21]. On the other hand, Lawless et al. [22] demonstrated in a recent systematic review that increasing up to 8 mm in interspace height during ACDF did not show a significant difference in fusion rate, postoperative Neck Disability Index (NDI), Visual Analogue Scale (VAS), and 12-item Short Form Health Survey score. Although there are no established results yet, the spacer size and height will affect the surgical outcomes of ACDF, and additional research is still needed on the exact relationship.
5) Plating
Cheung et al. [23] conducted a systematic review and meta-analysis to compare cage-only and traditional cageplate techniques. The cage-only technique is advantageous for reducing postoperative dysphagia, and adjacent segment disease (ASD) compared to the cage-plate technique. Meanwhile, the cage-plate technique is advantageous in reducing cage-subsidence and pseudarthrosis incidence rates, maintaining postoperative disc height, and restoring cervical lordosis [23]. Lee et al. [24] conducted a study on factors affecting subsidence after ACDF surgery. They confirmed that ACDF with plating lowers the incidence of subsidence postoperatively regardless of the kyphotic/lordotic cervical alignment preoperatively.
When comparing the types of cervical plates used, no significant difference was observed in the use of fixed-hole (static)-type plates and slotted-hole (dynamic) types for a single level. However, Nunley et al. [25] concluded that a dynamic-type plate is more effective in multilevel ACDF. In their study, although single-level ACDF was more effective than multilevel ACDF for pain relief, the VAS and NDI scores of the dynamic-type plate group were low in the multilevel ACDF.
6) Bone mineral density
Using biomechanical destructive compression tests, Lim et al. [26] reported that as bone mechanical strength was known to increase along with bone mineral density (BMD), vertebral BMD could be considered an essential factor in predicting the mechanical strength of the cage– endplate interface. Pinter et al. [27] investigated the role of BMD in patients while studying the risk factors for pseudarthrosis that may occur after ACDF. Data from 79 patients who underwent 1- to 4-level ACDF at a single research institute were retrospectively reviewed. At 1 year postoperatively, 65 patients (82%) achieved successful union; however, pseudarthrosis was confirmed in 14 patients (18%). When the pseudarthrosis subgroup was compared with the fusion subgroup, the pseudarthrosis subgroup demonstrated a significantly lower BMD (hip T-scores: −1.4±1.2 versus −0.2±1.2 and spine T-scores: −0.8±1.8 versus 0.6±1.9). Also, osteopenia, not osteoporosis, can increase the risk of pseudarthrosis after ACDF surgery. Hence, surgeons should consider the patients’ BMD when planning surgery [27].
7) Patient factors
Complete fusion of the segment after ACDF surgery is important for long-term prognosis. The surgeon should consider the instruments and techniques used during the surgery and the patients’ factors.
(1) Diabetes and smoking
Diabetes and smoking are worse prognostic factors that have been well-documented in the past. When comparing the frequency of non-union after ACDF surgery in patients with diabetes, the incidence of non-union was 1.7 times higher in the allograft group and 2.2 times higher in the cage group [8]. Cancienne et al. [28] reported that patients with diabetes are not a contraindication for single-level ACDF. Still, there is a significantly increased risk of deep postoperative infection when the perioperative hemoglobin A1c level exceeds 7.5 mg/dL [29].
Several studies have reported that tobacco use interferes with the bone union, and the incidence of non-union was approximately 2.6 times higher in the allograft group [8]. Cerier et al. [30] reported that smoking could affect the postoperative improvement in NDI scores in 2-level ACDF.
(2) Body mass index
Perez-Roman et al. studied the postoperative complication rate and surgical outcomes of ACDF associated with obesity status. In their analysis, obese patients with a body mass index (BMI) of 27.5 kg/m2 or more were found to have significantly higher rates of pulmonary embolism, wound infection, as well as dysphagia, neurological, cardiac, respiratory, hematological, and genitourinary complications [31]. Wang et al. [32] found that the patients who complained of immediate postoperative swallowing difficulties showed a higher BMI than the others (27.3 kg/m2 versus 24.6 kg/m2).
(3) Vitamin D
Vitamin D is well-documented to play an important role in bone metabolism. Some studies have confirmed that vitamin D deficiency can affect the outcomes of spinal fusion surgery. Khalooeifard et al. [33] conducted a meta-analysis and showed poor prognosis after surgery in patients with vitamin D deficiency (4.82 times Oswestry Disability Index increase).
3. Interesting topics for anterior cervical discectomy and fusion
1) Bone morphogenic protein in cervical spinal surgery.
In 2008, the Food and Drug Administration (FDA) warned against the use of bone morphogenic protein (BMP) for anterior cervical surgery. The overall complication rate was higher at 2.1% with BMP than without BMP (1.9%). More wound complications were also noted [36]. However, low-dose BMP has been used in contemporary clinical practice because of its several advantages. Mendenhall et al. [37] reported that using low doses of recombinant human BMP (rhBMP)-2 safely and effectively increased the fusion rate of anterior cervical fusion. In this study, 190 (96%) of 198 patients who received rhBMP-2 demonstrated firm joint union after 15 months. Additionally, the same level of coalescence in smokers, patients with multi-segmental ACDF, and those at high risk of pseudarthrosis was observed, suggesting that rhBMP-2 effectively increases the postoperative union rate [37]. The postoperative complication rate may increase when BMP is used to correct cervical deformity through an anterior surgical approach; however, the rate of subsequent revision surgery decreases [38,39].
2) Total uncovertebrectomy
(1) Literature
A technique of uncinate process resection during ACDF surgery was introduced and developed to achieve complete and direct decompression for severe bony foraminal stenosis. Total uncovertebrectomy originated from anterior cervical foraminotomy for unilateral cervical radiculopathy [40,41], and its safety and efficacy were evaluated in ACDF surgery [42]. In 2017, Lee et al. [43] conducted a study on the effectiveness and risk of uncinate resection during ACDF surgery. They reported that the uncinate resection group demonstrated faster clinical improvement than the non-uncinate resection group. Moreover, the fusion rates of both groups were comparable. Safaee et al. [44] have revealed detailed techniques of ACDF with uncinectomy and proved the safety and efficacy of direct anterior nerve root decompression. Among the 91 patients of myotome-specific weakness treated with uncinectomies, 80 (88%) improved, 10 (11%) had no change, and 1 (1%) worsened at the 6-week follow-up visit. Among 66 uncinectomies performed for dermatome-specific numbness, 57 (86%) were associated with complete resolution, and 9 (14%) demonstrated no improvement at 6 weeks. No patient developed worsening sensory symptoms [44]. Meanwhile, although a study has reported a significant correlation between uncinate process resection and subsidence during ACDF, Ferrete-Barroso et al. [45] have reported good results of anterior cervical arthrodesis using autograft bone originating from a total uncinate process resection.
(2) Surgical techniques
The critical point of this technique is the protection of the vertebral artery, which is located at the lateral aspect of the uncinate process. After dissection and coagulation of the longus coli muscle’s undersurface, the uncinate process’s lateral border is identified and gently detached from the soft tissue with the vertebral artery using a Penfield dissector. The end tip of the probe is maintained to avoid going deep and damaging the nerve root. Two uncinate resection techniques have been known. First, using a burr with a large matchstick head, the uncinate process is removed gradually from the inside at full height. If the lateral cortex of the uncinate process is sufficiently thin to move, the remaining fragment of the uncinate process can be easily fractured and removed. Second, using a slight head matchstick burr, the bottom of the uncinate process is removed from the medial side. If the remaining uncinate process is sufficiently removed to move, the whole fragment can be removed by breaking the left outer part using a small osteotome. The bone dust and fragment from the operation field can be used as bone grafts (Fig. 1).
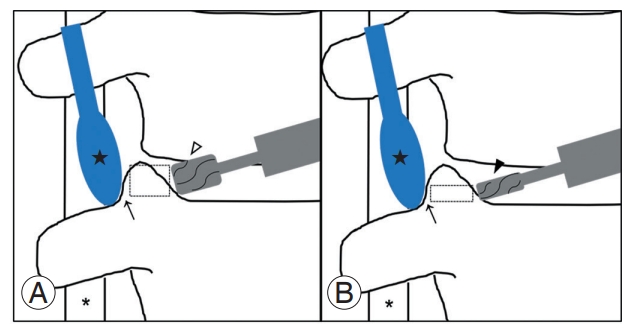
Scheme illustrating surgical techniques for total uncinate process resection. First, vertebral artery (*) is secured by gently placing the Penfield dissector (★) between the lateral margin of the uncinate process and soft tissue containing the vertebral artery. (A) Using a burr with large matchstick head (3.0 mm, ▷), the uncinate process is removed medially to laterally until the lateral wall becomes sufficiently thin (→) enough to be moved. (B) Using a burr with small head (1.8 mm, ▶), only the bottom of uncinate process is resected and the remained fragment can be easily removed without any complications (dotted rectangle: directly removed part by a burr).
4. Summary
Historically, ACDF surgery has been widely used in patients with cervical radiculopathy and has proven its safety. Many studies have been conducted to improve the prognosis of ACDF surgery. From what has been confirmed thus far, the allo-bone graft demonstrates better results than other spacers. Additional research on the effectiveness of cages is needed, but the prognosis of PEEK and Ti cages is relatively favorable to date. During spacer insertion, using a cage with a cage/endplate ratio of ≥65% and positioning it close to the anterior cortical bone helps to lower the subsidence rate. The use of a plate has the possibility of complications caused by the plate, although it may decrease subsidence. Thus, the pros and cons of its use should be considered. The preoperative conditions of patients should also be considered. Determining bone quality, the presence of diabetes, and smoking status in advance is crucial as such factors can affect the postoperative prognosis. There has been controversy over the use of rhBMP-2, but recently, a method of using rhBMP-2 at a low dose to reduce side effects has been attempted in consideration of the advantages of rhBMP-2.
Cervical Disc Replacement
To date, the most common surgical method for cervical disc herniation is ACDF, but it has a limitation that causes ASD [46]. Therefore, CDR was devised as a surgical procedure to preserve joint mobility [47,48].
1. Indication and contraindication
The indications for surgery approved by the FDA include level 1 or 2 segment cervical degenerative diseases or disc herniation and foraminal osteophytes that cause radiculopathy or myelopathy in adult patients [49,50]. Contraindications include osteoporosis or metabolic bone disease, active or prior infection, ankylosis, congenital cervical stenosis, facet arthritis, and segmental cervical instability. Pimenta et al. [51] presented instability as an exclusion criterion for CDR in a study comparing the outcome of multilevel cervical arthroplasty with single-level surgery, which was defined as anterior subluxation of 3.5 mm or more in flexion-extension radiographs. Additionally, because of the stability of the prosthesis, the posterior structure must be structurally and functionally intact. Therefore, previous laminectomy or excessive facet removal is contraindicated [49]. Ossification of the posterior longitudinal ligament (PLL), renal failure, cancer, rheumatoid arthritis, diffuse idiopathic skeletal hyperostosis, and preoperative corticosteroid medication should also be avoided [52].
2. Factors for better surgical outcomes
1) Implant type
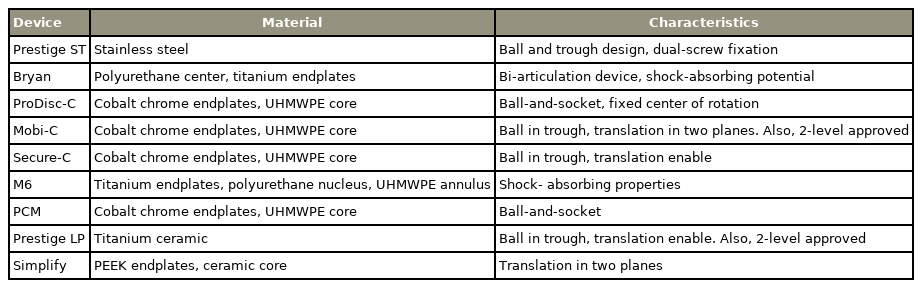
CDR was devised to reduce the occurrence of ASD during long-term follow-up by maintaining the motion of the cervical spine, and it can be categorized as constrained, unconstrained, or semi-constrained. The term constraint refers to the kinematic degree of freedom of an object in a 3D space in biomechanics and is classified as above according to the degree of motion in a CDR device [53]. The unconstrained types include the Bryan, Mobi-C, PCM, and Prestige LP, which may cause sagittal malalignment, including kyphosis or hypermobility due to lack of constraint. The semi-constrained types include the ProDiscC, Prestige ST, Simplify, M6, and Secure-C, which are known to demonstrate similar results to those of the unconstrained types. Recently, a patient-specific device using 3D printing has been introduced, which may have a better effect on patient outcomes in the future [49,54].
(1) Unconstrained types
The Bryan disc is a bi-articulation device comprising a polyurethane center and two Ti endplates, which induce bony ingrowth through a porous surface and provide stability. This prevents posterior dislocation of the device through the anterior flange. The polyurethane center is enclosed with a saline-filled sheath, which forms a pseudocapsule, and the cushioning effect of the actual disc is expected [49]. The Mobi-C disc comprises three components. The two endplates were made of cobalt, chromium, and 29 molybdenum ISO (International Organization for Standardization) 5832-12 alloy, and each line with teeth was on its outer edge. The internal contact surface of the inferior end plate is spherical, and the superior end plate is flat [49,55].
(2) Semi-constrained types
The ProDisc-C disc comprises two components of ball- and-socket, and the porous endplate is made of cobalt chrome. The medial surface of the inferior endplate is convex, and the insert surface articulating with the superior endplate is concave; therefore, translation was limited, and rotation was allowed in all three axes. The outer surface of the implant was coated with Ti [49]. Prestige ST has a ball-and-trough design, and dual-screw fixation is performed on each adjacent vertebral body. It is composed of stainless steel containing iron, carbon, nickel, molybdenum, and chromium [49,56]. The M6 disc is a single piece with a complex centerpiece composed of Ti alloy endplates and polycarbonate urethane polymeric material. This material serves as the nucleus pulposus and is surrounded by polyethylene woven fibers that serve as the annulus fibrosis. The polymer sheath prevents infiltration of debris and ingrowth of tissues [49]. The Secure-C disc comprises three components. The two endplates were keeled porous-coated cobalt chrome with an ultrahigh molecular weight polyethylene sliding center. The upper side of the center was spherical, whereas the lower side was cylindrical. This allowed for anterior–posterior (AP) sliding and physiological loading, simultaneously causing movement of the axis of rotation in the sagittal plane. It also prevents the core from leaving and protecting the facet from excessive loading [49,57] (Table 2).
2) Implant size and height
Selecting the appropriate size, shape, and height of the artificial disc can produce good results for the patient; however, size and height are critical. Suppose the height of the device is approximate ≥2 mm than the standard value. In that case, it can cause a significant change in the stress of the cervical biomechanics and bone-implant interface, which can cause ASD or subsidence [58]. The device’s height is higher than normal; massive stress is applied to the disc, resulting in a shortened lifespan. However, if the device’s height is short, cervical kyphosis or a decrease in flexion motion may occur. Therefore, adjusting the device’s size is necessary to prevent compressing the spinal canal or exceeding the anterior margin of the disc space. Additionally, if the device is small, early subsidence or stress concentration on the small surface may cause early wear. Thus, a device that can cover the endplate within 1–2 mm of the original vertebral body should be selected [59]. The appropriate implant size is finally determined through trial, and the AP and lateral images are checked. In many patients, the uncus can be asymmetrical, in which case the implant can shift laterally, even if it fits into the midline of the disc. In this case, one can select a smaller implant, remove the protruding uncus, and repeat the procedure [59,60].
3) Location of implant
After applying the implant to the disc space, fluoroscopic imaging should confirm proper positioning [54,61]. The proper position of the device in the coronal plane is to check the line connecting the spinous process that is projected with the center of the stone connecting the unit on fluoroscopy. In the sagittal plane, the device is placed perpendicular to the posterior wall of the vertebral body at the level to be operated on, passing through the center of rotation (COR) of the CDR [62].
4) Preservation or removal of the posterior longitudinal ligament
The preservation and removal of PLL during CDR surgery in patients with cervical radiculopathy remain controversial. Depending on the surgeon’s choice, the PLL can be removed or released, which can be influenced by device type. There are advantages and disadvantages to preserving and removing the PLL. The hypotheses that preserving the PLL can maintain adequate cervical ROM and that removing the PLL can cause hypermobility are conflicting [54]. Voronov et al. [63] have suggested that hypermobility can be solved by applying a stiffer device during device implantation. Kim et al. [64] also mentioned that PLL is already damaged in case of severe herniation of the disc material, so it is removed only in this case. Some surgeons consider PLL removal the best means to ensure sufficient decompression [54].
5) Patient factors
Selecting an appropriate patient group is important to attain a good prognosis in CDR. Patients with one or two-segment radiculopathy or myelopathy caused by soft disc herniation with physiological motion have an excellent postoperative prognosis. Selecting patients without osteoporosis, hypermobility, kyphosis, or scoliosis is advantageous [59].
(1) Smoking
Tu et al. [65] compared clinical and radiologic outcomes by dividing 109 patients who underwent CDR into the smoking group (20 patients) and the non-smoking group (89 patients). In this study, while no significant difference in clinical outcomes was observed between the two groups, a significant difference in segmental ROM in the smoking group was observed (8.1°→8.1° versus 8.2°→6.9°). Although no significant difference in the generation of heterotopic ossifications (HO) (50.0% versus 59.6%) was observed, the smoking group demonstrated a relatively low incidence rate [65].
(2) Others
Zeidan et al. [66] analyzed factors affecting readmission or prolonged length of stay after CDR. The factors influencing readmission were postoperative superficial wound infection (OR, 73.83), American Society of Anesthesiologists (ASA) classification (OR, 1.98), and BMI (OR, 1.06). The factors affecting the prolonged length of stay were female sex (OR, 1.76), diabetes (OR, 1.50), postoperative wound dehiscence (OR, 13.11), ASA class (OR, 1.43), and operative time (OR, 1.01) [66].
3. Surgical techniques: proper patient’s position and sizing and settling of the implant
The patient is placed in a supine position with the head in a neutral position and is secured to the operating bed with tape on the forehead to prevent the head from moving during the operation. To prevent the shoulder from overlapping with the cervical vertebrae during radiography, the tape is attached and fixed by pulling it toward the patient’s foot. Also, position the patient so the end plate of the area can be operated on in parallel [67].
To prevent the formation of HO, a unique complication of cervical arthroplasty, the use of drills should be reduced as much as possible [49,67]. In CDR, implant sizing and centering are the most important factors [68]. Under a fluoroscopic device’s operation, an appropriate size implant is selected and inserted. At this time, centering is confirmed through the AP and lateral radiographs [67]. Placing the device’s COR slightly behind the midline of the intervertebral disc space provides a better combination of the actual segment’s COR and the device’s COR. Many surgeons prefer this because it can reduce the occurrence of subsidence. After all, the strong posterior rim of the end plate holds up [61,69].
4. Interesting topics for cervical disc replacement
1) Hybrid surgery
Recently, the number of hybrid surgeries in which ACDF and CDR are performed together has increased in patients with multilevel cervical degenerative disc disease [70]. This is suggested when it is challenging to apply fusion or arthroplasty equally to all levels because the degree of degeneration at each level is different. This is known to limit the hypermobility of adjacent segments while maintaining the segmental motion of each segment, and several studies have reported better results with combined ACDF and CDR than with ACDF or CDR alone. Moreover, the postoperative assessment, complication rate, and functional score have been reported to be excellent or similar. Whether the arthroplasty level was above or below the fusion level did not indicate a significant difference in motion, pressure applied to the adjacent disc, or facet joint force [71,72]. Jia et al. [71] have reported that complications included dysphasia, heterotropic ossification, and vocal cord paralysis during hybrid surgery, and no non-union occurred. This is expected to cause less non-union as more ROM remains in the segment during hybrid surgery, which has the effect of applying less stress to the adjacent level. A comparative study should be conducted to determine whether hybrid surgery has fewer non-union occurrences than ACDF does.
2) Long-term results of cervical disc replacement
Compared with ACDF, CDR has a lower reported incidence of ASD and a lower reoperation rate due to improved ROM. However, some studies have reported that ROM may decrease owing to heterotropic ossification, mechanical malfunction of the device, or implant wear. As a result of comparing several studies, Hence, Zavras et al. [73] reported that ROM immediately postoperatively improved compared to ROM preoperatively, and no significant change in follow-up up to 1 year was observed. However, in the long-term follow-up (average, 99.86 months), the ROM deteriorated significantly, and efforts to maintain a good long-term ROM were necessary [73].
Burkus et al. [74] confirmed the 7-year outcome of the CDR group using the Prestige ST device, and all demonstrated promising results. Especially when compared to ACDF, NDI score, neurologic status, and revision rate due to ASD have good results. A study by Gornet et al. [55] also demonstrated a similar conclusion to the abovementioned study comparing the ACDF group in the 7-year outcome after CDR using the Prestige.
5. Summary
CDR is an operative treatment for patients with cervical radiculopathy that reduces the incidence of ASD during long-term follow-up by maintaining cervical spine motion postoperatively. Various devices classified as constrained, unconstrained, or semi-constrained are selected according to the patient’s condition and the operator’s preferences. Selecting an artificial disc of an appropriate size, shape, and height and inserting it into an ideal position is a decisive factor in achieving a good patient prognosis. Selecting patients with level 1 or 2 segments of cervical degenerative disease or disc herniation who do not correspond to the abovementioned contraindications is also essential. Recently, topics of interest have focused on hybrid surgery, which involves performing ACDF and CDR together, and the long-term consequences of the CDR.
Posterior Cervical Foraminotomy
PCF is a safe and effective surgical method for treating patients with cervical radiculopathy [75,76]. PCF avoids complications of anterior surgery, such as postoperative hematoma, dysphagia, and recurrent laryngeal nerve palsy [75,77]. Additionally, since fusion is not required, fusion-related side effects such as pseudoarthrosis and instrumentation failure do not occur. However, its disadvantage is that considerable posterior muscle damage is possible, and the rate of reoperation is higher than that of ACDF because fusion is not performed [78-80].
1. Indication and contraindication
The indications for PCF are relatively narrow compared with those of the other surgical methods. It is commonly performed in patients with level 1 or 2 unilateral radiculopathies [81]. Moreover, it is appropriate only when foraminal stenosis is clearly confirmed on magnetic resonance imaging and when nerve root compression caused by soft lateral disk herniation and corresponding radicular symptoms are present [82]. Meanwhile, if cervical instability is confirmed by stress X-ray, PCF is contraindicated. Additionally, myelopathy or central stenosis is caused by extensive spondylotic disease and diseases caused by comprehensive anterior disc or osteophytes [81-83].
2. Factors for better surgical outcomes
To improve the postoperative prognosis of patients, selecting the right patient for PCF is important. The main symptoms, such as arm and neck pain, are generally reduced postoperatively [82]. Skovrlj et al. [84] have reported significant improvements in both NDI and VAS postoperatively compared to that preoperatively, and Church et al. [85] have reported >85% improvement in pain, weakness, and function in a 10-year follow-up study postoperatively. Witzmann et al. [86] showed excellent functional and economic results in 90% of patients. The study by Holly et al. [87], comparing the group that underwent multilevel surgery and the group that underwent single-level surgery, showed similar results. Meanwhile, in a large cohort study by Church et al. [85], they concluded that radiculopathy due to soft disc subtypes might be associated with a better prognosis compared to osteophyte disease, although osteophyte disease remained an excellent indication for PCF.
3. Detailed techniques of posterior foraminotomy
Since Zdeblick et al. [88], in their biomechanical study, had demonstrated that torsional stiffness dramatically decreased when ≥50% of the facet was removed, and posterior strain significantly increased at 75% or 100% facet resection compared to the intact case, resection of ≥50% of the facet was considered to cause cervical hypermobility. The first step in PCF is to resect the medial 1/2 of the facet joint to decompress the cervical nerve root [89]. Accurately marking the medial 1/2 of the facet joint is necessary. In a cadaveric study, Barakat and Hussein [90] reported that the horizontal distance from the medial point of the facet joint to the lateral surface of the dura after medial 1/2 facetectomy was 7.1–9.8 mm, and Hwang et al. [91] mentioned that the mean distance of the exposed root was 8.2–9.0 mm. Therefore, approximately <10 mm (mediolateral) laminoforaminotomy is considered a proper length. To avoid cervical instability, the facet should be resected to <50%, and for this, removing up to 5 mm of the lateral mass is considered safe [92-95].
When decompressing the nerve root, identifying the difference in the anatomical relationship between the nerve root and disc at each cervical level is necessary. In particular, in the case of C8, the frequency of radiculopathy is relatively lower than those of the other levels due to the transverse course of the nerve root. Therefore, when performing decompression of the C8 nerve root, more extensive decompression should be performed on the proximal and lateral parts [96].
During PCF, the most technically demanding aspect is bleeding control for a clear view. To decrease intraoperative bleeding and minimize facet joint damage, the authors usually perform the PCF using an en-bloc resection technique. This technique proceeds in the following order. First, the inferior articular process is removed, and a cutting groove is made at the superior articular process (SAP), which proceeds from the transverse direction to the vertical direction. After making shallow transverse and vertical grooves in the medial half of the SAP, this cutting groove is made more profound, and then the bony fragment is twisted using a small osteotome. Because we can hear a clicking sound, this technique is called the “click method.” Finally, the en-bloc fragment can be detached and removed using a micro-curette (Fig. 2).
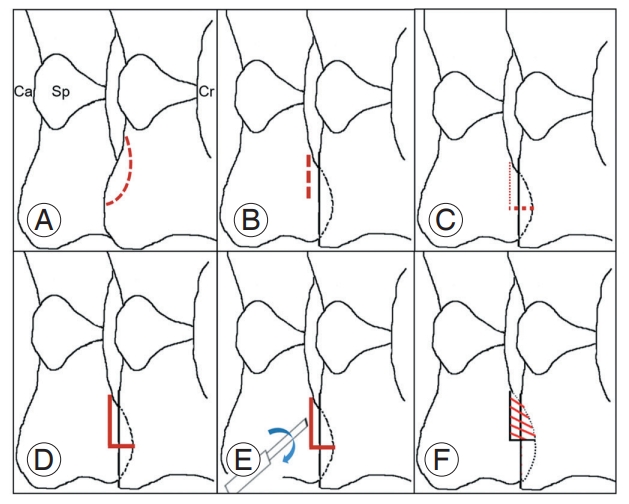
Scheme illustrating surgical techniques for posterior cervical foraminotomy using en-bloc resection. (A) Inferior articular process removal, (B) superior articular process (SAP)-transverse cutting groove, (C) SAP vertical cutting groove, (D) groove deepening, (E) fragment twisting with a small osteotome, named ‘click method,’ and (F) fragment detaching and removal with a microcurette. Ca, caudal; Sp, spinous process; Cr, cranial.
4. Interesting topics for posterior foraminotomy
1) Endoscopy
PCF using endoscopy is widely performed. This method demonstrates clinical and radiological results similar to the open technique and has the advantage of minimizing complications compared to the open technique [97-99]. Additionally, studies have reported reduced surgery time, shortened hospitalization period, and quicker return to daily life [100-102]. The surgical indication for cervical foraminotomy using endoscopy is similar to the conventional method, although this should be avoided when the spinal cord is compressed [103]. Recently, anterior foraminotomy with endoscopy has been performed in patients with cervical radiculopathy to protect the intervertebral disc and maintain the functional motion segment during surgery. Kabil and Abdel-ghany [104] performed microendoscopic anterior cervical foraminotomy in patients with unilateral radiculopathy with single levels of soft disc herniation or hard disc osteopathy and have reported the clinical outcomes. In this study, patients demonstrated excellent postoperative results in most clinical outcomes, including neck pain and arm pain, were able to perform neck movements immediately after surgery and did not require cervical collars.
2) Posterior foraminotomy with laminoplasty
Sasai et al. [105] have reported that microsurgical PCF with laminoplasty produced good results in patients with cervical spondylotic radiculomyelopathy. Lee et al. [106] and Liu et al. [107] have also reported that laminoplasty with PCF could be an efficient and safe treatment for patients with combined myelopathy and radiculopathy. Komagata et al. [108] have reported that performing expansive laminoplasty and foraminotomy simultaneously in patients with cervical spondylotic myelopathy or ossification of the PLL helped prevent C5 palsy. Recently, the importance of this PCF procedure may be increasing in patients who undergo laminoplasty for cervical myeloradiculopathy.
3) Long-term results
Heo et al. [109] performed a long-term follow-up of 46 patients who underwent PCF surgery for 74.4 months. Among them, 39 (84.7%) and 25 (71.4%) showed improvement in radiculopathy and neck pain, respectively, and excellent clinical results were confirmed. Bielecki M et al. [110] followed up 48 patients who underwent PCF surgery for 100.8 months and confirmed that the NRS arm, NRS neck, and NDI scores improved by 7, 4.5, and 24 points, respectively, and reported that radiological results also improved in 82%.
MacDowall et al. [111] have reported in a review article that, as a result of comparing the PCF group of 647 patients and the ACDF group of 3,721 patients, there was no significant difference in NDI scores, EuroQol-5 dimension, and VAS for neck and arm scores at 5 years after surgery. From this, it can be assumed that PCF surgery shows excellent clinical and radiological results in long-term follow-up. Its excellence is not inferior even when compared with ACDF [111].
5. Summary
PCF is a safe and effective surgical method to avoid complications associated with fusion and possible anterior surgery in patients with cervical radiculopathy. The indications for PCF are relatively narrow compared to those of other surgical methods, commonly in patients with level 1 or 2 unilateral radiculopathies. Recently, endoscopic surgery, which can minimize complications compared to open surgery, has attracted increasing attention. Endoscopic surgery reduces operative time, shortens the length of hospital stay, and expedites the return to daily life. Long-term follow-up of PCF surgery shows excellent clinical and radiological results, and its excellence seems not to be inferior to other surgical methods.
ACDF versus CDR versus PCF
1. ACDF versus CDR
Findlay et al. [112], in a review study of 22 papers with 3,160 patients, compared long-term outcomes after ACDF and CDR and found no significant difference between the two groups in short-term outcomes up to 2 years. However, in the 4th and 7th-year comparisons, the CDR group was superior to the ACDF group in most items, such as NDI, 36-item Short Form Health Survey, dysphagia, and satisfaction (overall success results favored CDR; relative risk=0.60) [112]. Comparing the postoperative ASD incidence, in most cases, the CDR group showed a lower incidence of ASD than the ACDF group. In particular, in a study by Zhong et al. [113], the incidence of ASD in the CDR group was significantly smaller than that in the ACDF group (3.0% versus 8.0%). In a 7-year follow-up study by Janssen et al. [114], the incidence of ASD in the CDR group was significantly smaller than that in the ACDF group (5.8% versus 12.2%). To date, particularly in medium- to long-term follow-up, the rate of symptomatic ASD seems to be lower in the CDR group than in the ACDF group [55,74,113-125].
2. ACDF versus PCF
MacDowall et al. [111] compared the outcomes of ACDF and PCF in a population-based large cohort (4,368 patients). Although they have reported that both groups demonstrated clinical improvements at the 5-year follow-up that did not achieve a clinically significant difference from one another, the secondary surgeries on the index level due to restenosis were more frequent in the foraminotomy group and on the adjacent segments, there was no difference between groups. In a study comparing minimally invasive PCF using tubes and ACDF, Dunn et al. [126] showed no difference in overall revision proportion between the two groups. Also, there was no significant difference in NDI and VAS scores before and after surgery. In a select group of patients, PCF and ACDF demonstrated similar surgical outcomes to those of surgical treatments for cervical radiculopathy.
3. Reoperation
The main issue of CDR is to leave motion of the cervical spine. Therefore, good candidates for CDR are young patients whose basic cervical spine movements are well preserved. When referring to knee and hip artificial joint surgery, the survival rate of artificial joints is 91% at 10 years and 78% at 20 years [127]. It can be interpreted that young patients will undergo revision surgery at least once in their lifetime. Joaquim et al. [128] conducted a systematic review on patients undergoing reoperation due to CDA failure. During the 5-year follow-up period, the CDA failure rate at the index level was 3.9%, and ACDF was the most frequently performed salvage surgery for failed CDA. Reasons for reoperation include worsening of symptoms, persistent neck or arm pain, disc subluxation, postoperative myelopathy, oversized implant, mal-positioned implant, and iatrogenic fractures of the posterior vertebral wall [128].
Wang et al. [129] investigated 178 patients who underwent PCF surgery for 31.7 months and found that only 9 (5%) received revision ACDF in the segment where PCF was performed. Patients who received revision ACDF were younger on average (25 years versus 35 years), had a lower BMI (25 kg/m2 versus 29 kg/m2), and were more likely to take anti-anxiety (56% versus 22%) or antidepressants (67% versus 27%) medications compared to those who did not undergo revision ACDF surgery [129]. Lubelski et al. [75] compared the revision surgery rate of ACDF and PCF within 2 years of the initial surgery, and there is no significant difference between them (4.8% for ACDF versus 6.4% for PCF). Meanwhile, suppose a patient complains of radicular symptoms after CDR. In that case, the implant is positioned well, the canal is decompressed well, and the radicular symptoms derived from foraminal stenosis or disc herniation, PCF can be performed. The appropriate subjects for this operation are the patients who show Spurling signs positive and symptoms of improvement when the neck flex forward. The Spurling method narrows the foramen, causing symptoms of nerve compression, and the forward flexion plays a role in alleviating the symptoms by expanding the size of the foramen [130].
Conclusions
Numerous studies on patients with cervical radiculopathy have been performed, but the data on analyzing factors for better surgical outcomes are still confusing. In this study, a detailed description of three surgical methods for patients with cervical radiculopathy and a comprehensive analysis of factors for better surgical outcomes were conducted. In the ACDF surgery, allo-bone graft, cage implants, spacer location, spacer size and height, plating, BMD, and other factors (diabetes and smoking, BMI, vitamin D, steroid use) can be important factors for better surgical outcomes. In the CDR surgery, type, size, height, location of the implant, PLL preservation or not, and other patient factors (smoking, ASA classification, BMI, etc.) are considered significant prognostic factors. In PCF surgery, the patient’s selection for proper indications and subtypes of the soft or hard disc is critical for postoperative surgical outcomes.
Most of the recent literature demonstrated that all three surgical techniques (ACDF, CDR, and PCF) for patients with cervical radiculopathy have clear advantages and disadvantages and reveal satisfactory surgical outcomes under a proper selection of patients and application of appropriate surgical methods. Meanwhile, exciting topics for the ACDF surgery include the usage of BMP and total uncovertebrectomy or not, and interest in the long-term results of the CDR surgery compared to the ACDF and hybrid surgery (combined surgery of ACDF and CDR) is increasing. In PCF surgery, endoscopic surgery and long-term results are recently interesting. For this, it is important to fully understand the factors for better surgical outcomes and to adequately practice surgical techniques for patients with cervical radiculopathy.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: KCK and TSJ; data curation: KCK and CHJ; funding acquisition: KCK; methodology: all authors; project administration: KCK; visualization: KCK; writing–original draft: all authors; writing–review & editing: all authors; and final approval of the manuscript: all authors.