 |
 |
- Search
| Asian Spine J > Volume 17(2); 2023 > Article |
|
Abstract
Notes
Funding
This study was supported by the National Natural Science Foundation of China (No. 81760231; No. 81160220); Natural Science Foundation of Ningxia Hui Autonomous Region (CN) (No. NZ17153; No. NZ11197); First-Class Discipline Construction Founded Project of NingXia Medical University and the School of Clinical Medicine (NXYLXK2017A05); and Natural Science Foundation of Ningxia Hui Autonomous Region (No. 2022AAC03398).
Author Contributions
Conception and design: Li Junjie, Yuan Haifeng; data acquisition: Li Junjie, Yin Jiheng, Liu Jun; analysis of data: Li Junjie, Yin Jiheng, Liu Jun; drafting of the manuscript: Li Junjie; critical revision: Li Junjie, Yin Jiheng, Liu Jun, Yuan Haifeng; obtaining funding: Lin haixiong, Yuan Haifeng; administrative support: Lin haixiong, Yuan Haifeng; and supervision: Yuan Haifeng.
Fig.┬Ā1
Fig.┬Ā2

Fig.┬Ā3
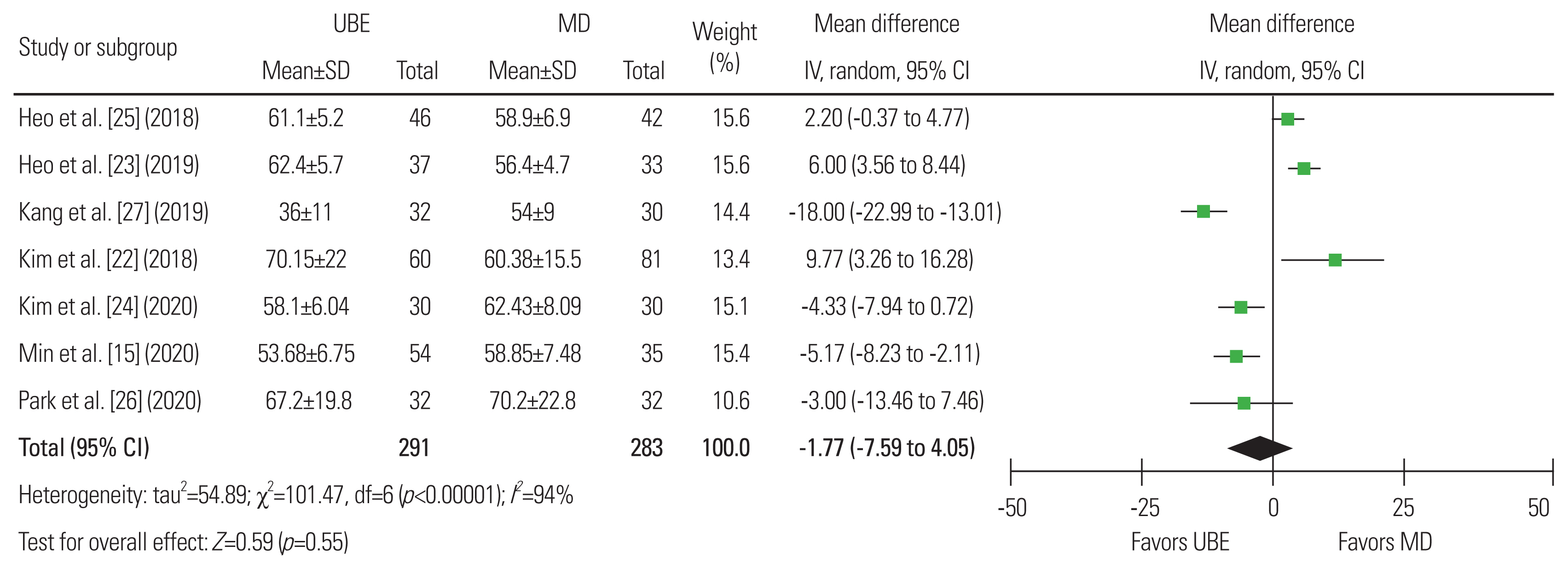
Fig.┬Ā4
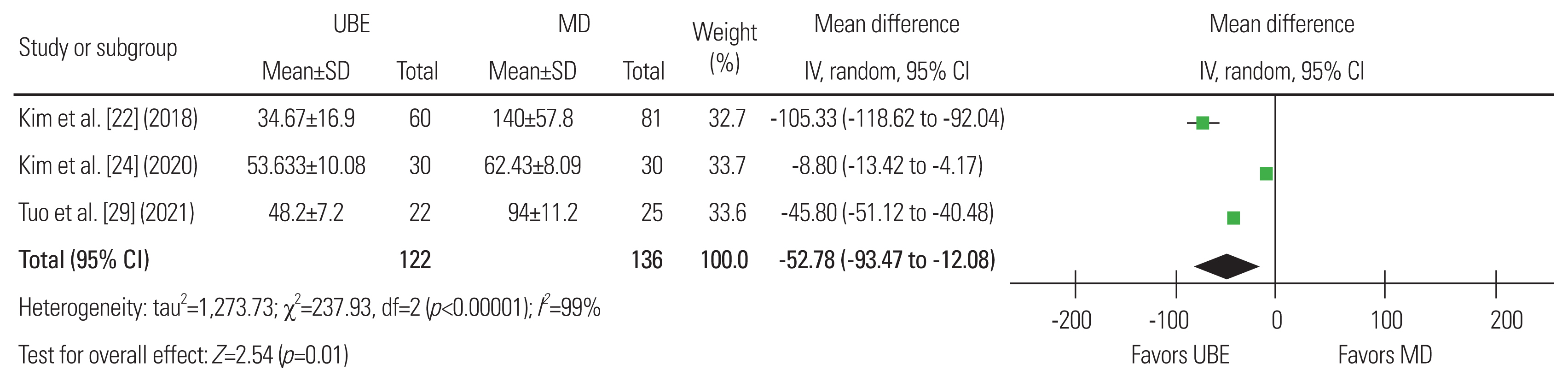
Fig.┬Ā5
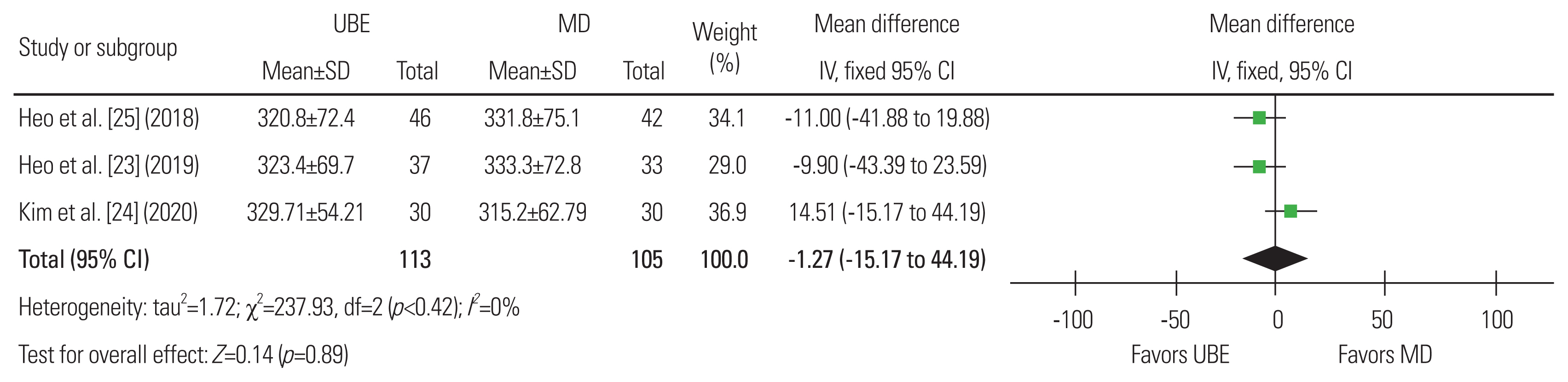
Fig.┬Ā6
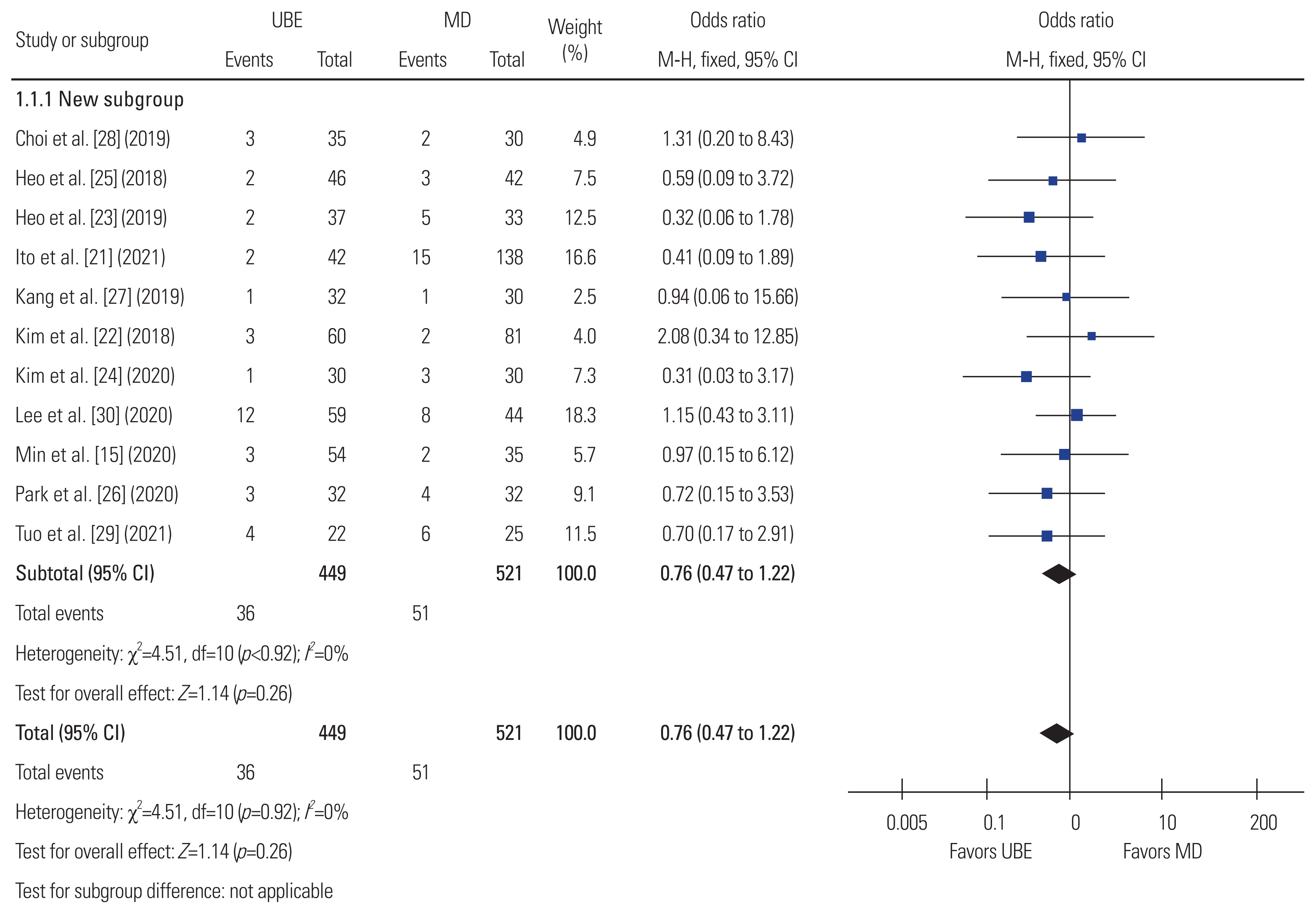
Fig.┬Ā7
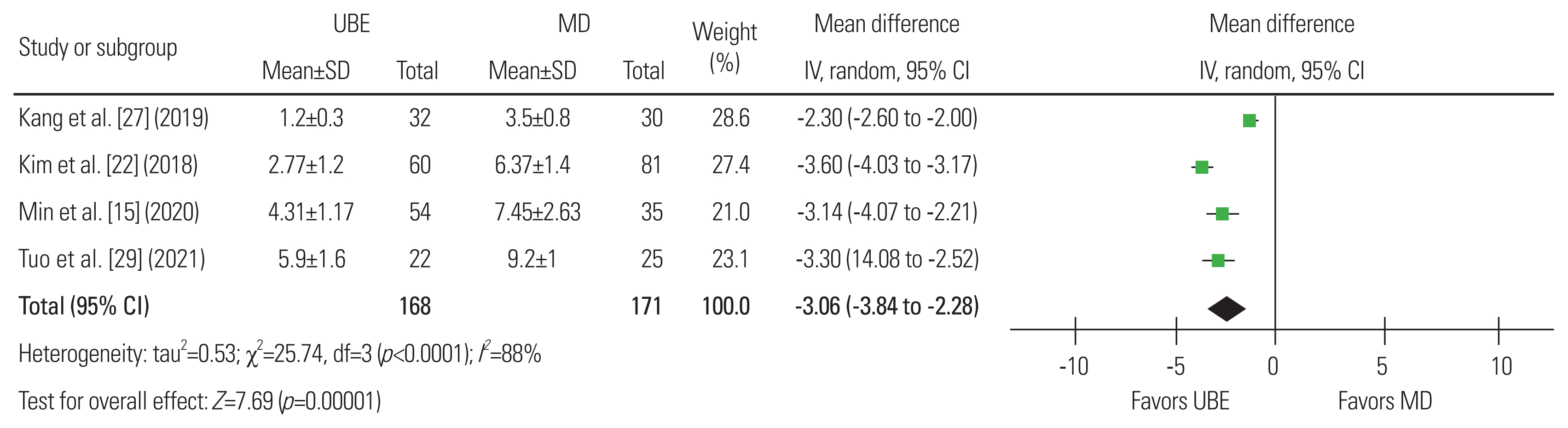
Fig.┬Ā8
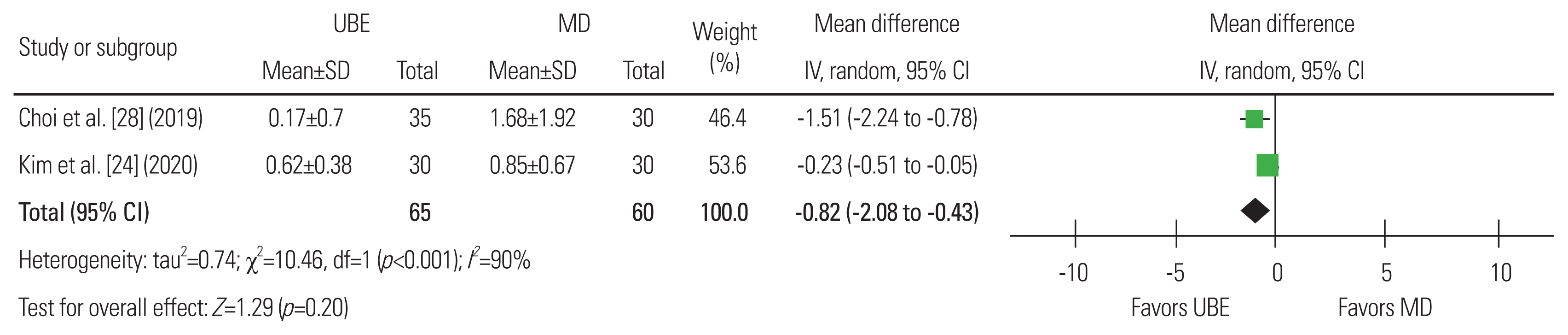
Fig.┬Ā9

Fig.┬Ā10
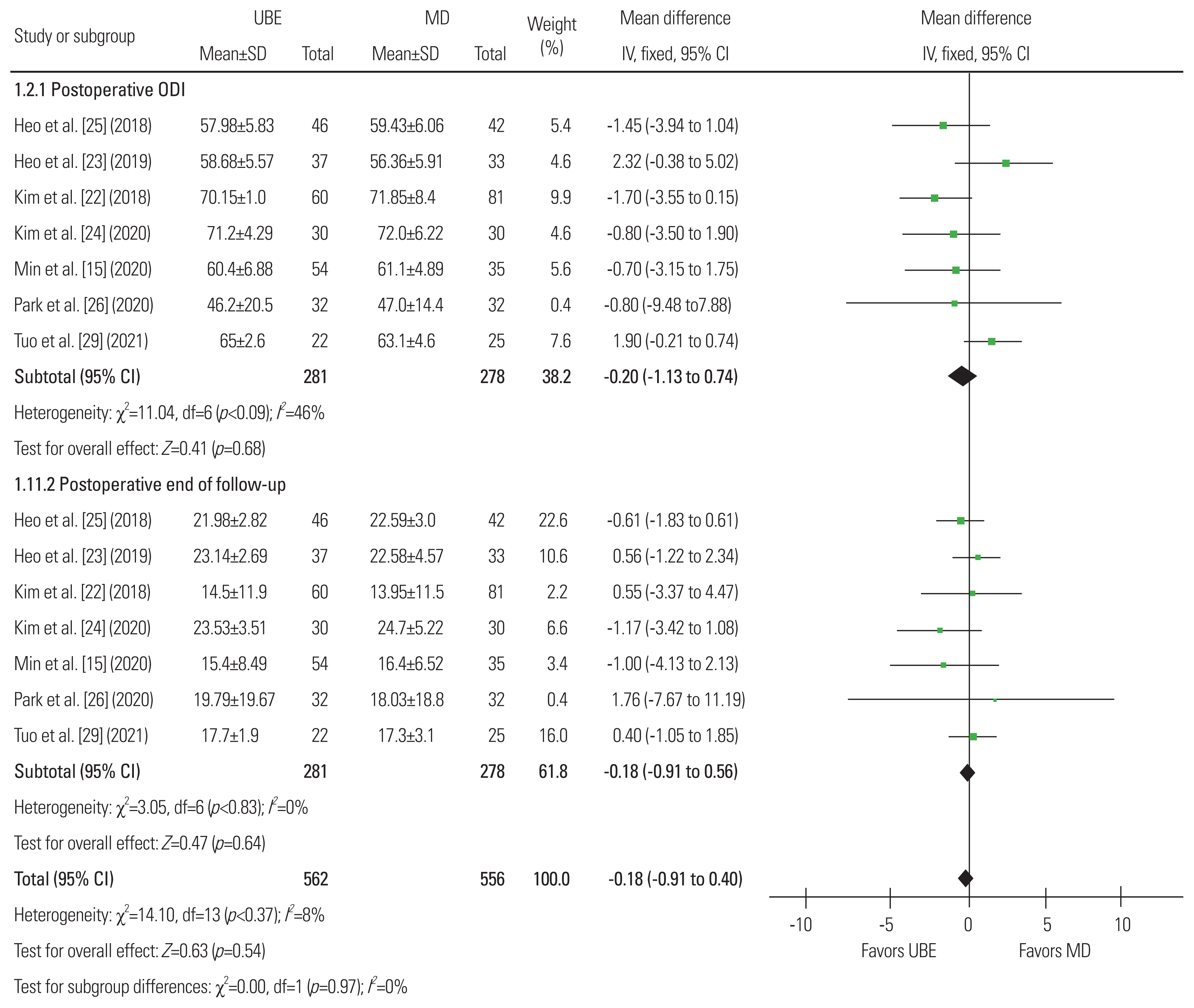
Fig.┬Ā11
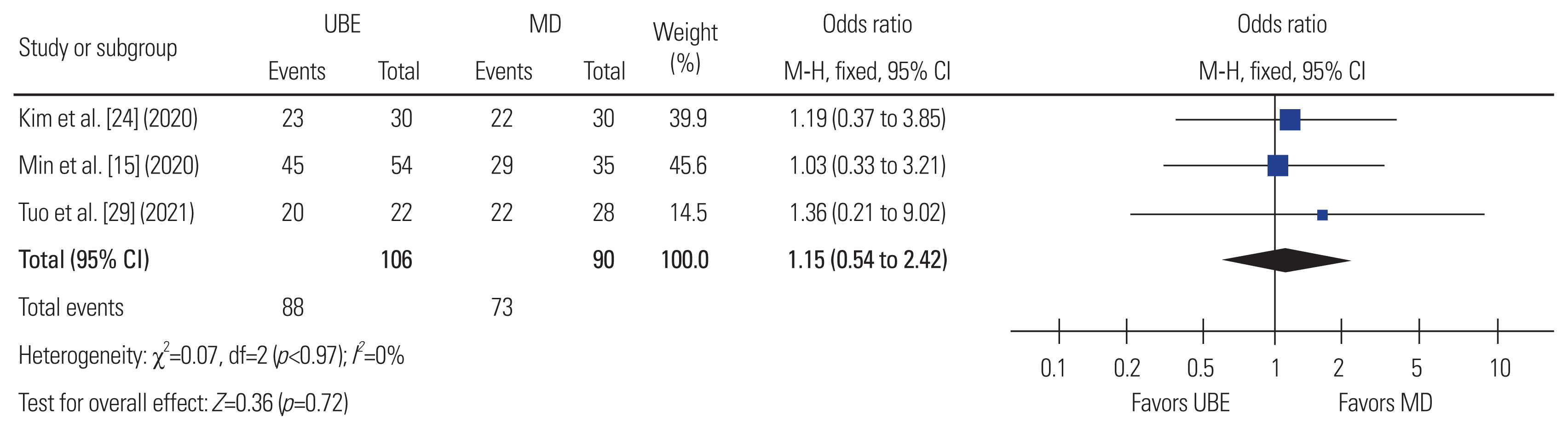
Fig.┬Ā12
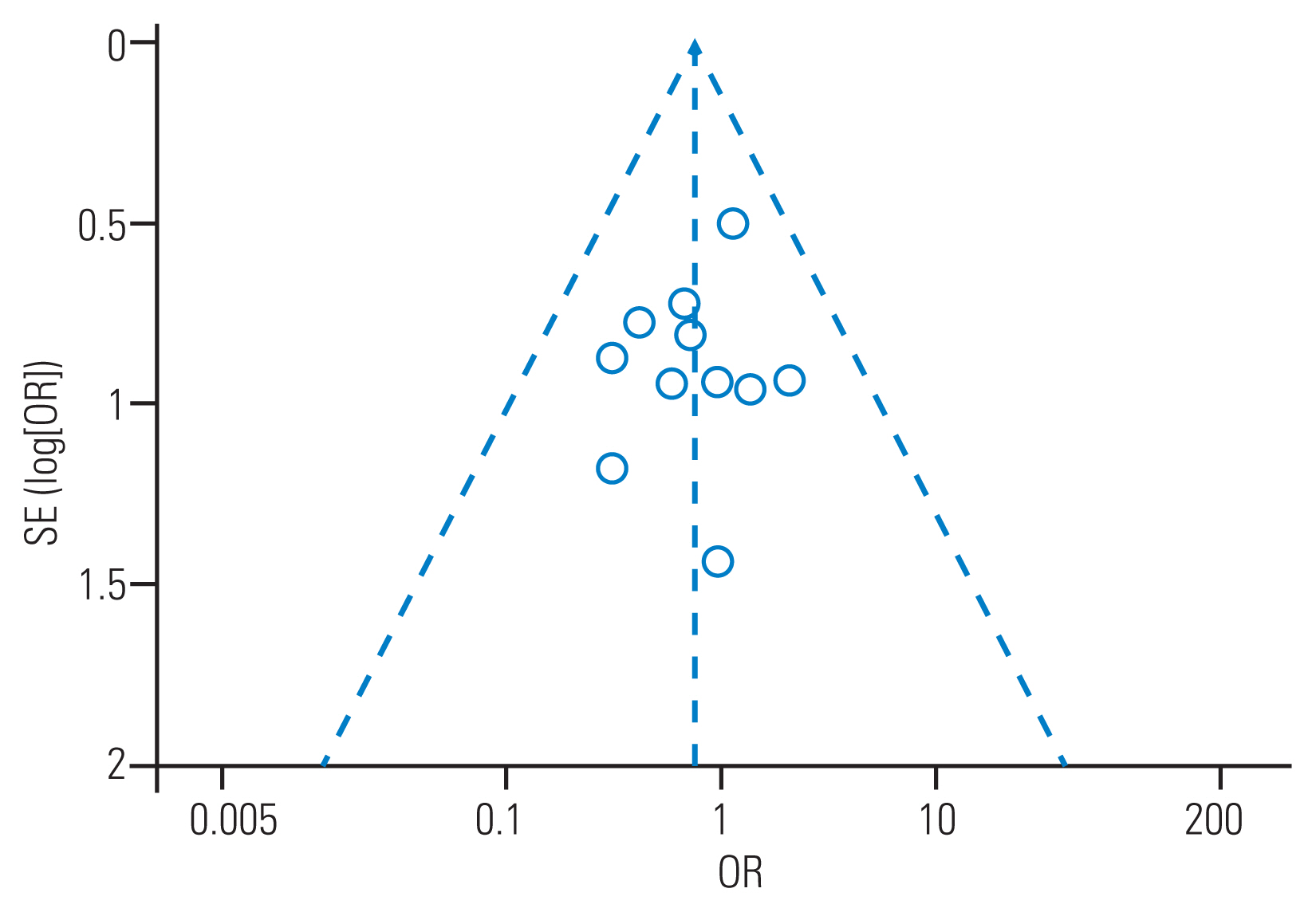
Table┬Ā1
| Study | Design | Country | No. of patients (UBE/MD) | Age (yr) (UBE/MD) | Sex (M:F), (UBE/MD) | No. of levels | Complications | Outcomes | Final follow-up (mo) |
|---|---|---|---|---|---|---|---|---|---|
| Min et al. [15] (2020) | Case control study | Korea | 54/35 | 65.74┬▒10.52/66.74┬▒7.96 | 27:27/19:16 | All single level | UBE: dural tear (n=2), postoperative epidural hematoma (n=1); MD: dural tear (n=1), postoperative epidural hematoma (n=1) | VAS for back and leg; ODI; operative time; modified Macnab criteria; complication |
UBE: 27.2┬▒5.4 MD: 31.5┬▒7.3 |
| Ito et al. [21] (2021) | Single-center, retrospective analysis | Japan | 42/139 | 66.3┬▒12.3/65.0┬▒11.1 | 28:14/71:68 | All single level | UBE: dural injury (n=2); MD: hematoma (n=5), dural injury (n=8), reoperation (n=2) | VAS for back and leg; ODI; operative time; bone resection area; facet; preservation rates; EQ-5D questionnaire; complication |
UBE: 6.7┬▒0.6 MD: 6.9┬▒0.8 |
| Kim et al. [22] (2018) | Multicenter, retrospective analysis | Korea | 60/81 | 46.60┬▒14.18/54.22┬▒20.21 | 37:23/24:57 | All single level | UBE: change the surgical plan (n=3); MD: cerebrospinal fluid leakage (n=1), cerebrospinal fluid leakage and infection (n=1) | VAS for back and leg; ODI; operative time; complication; estimated blood loss; hospital stay; MacNab score; complications |
UBE: 12.60┬▒1.03 MD: 12.84┬▒1.30 |
| Heo et al. [23] (2019) | Retrospective analysis of prospectively | Korea | 37/33 | 66.7┬▒9.4/63.4┬▒11.1 | 15:22/12:21 | All single level | UBE: durotomy (n=1), postoperative hematoma (n=1) MD: durotomy (n=2), transient weakness (n=1), postoperative hematoma (n=2) | VAS for back and leg; ODI; operative time; dura expansion; angle of facetecomy; complication |
UBE: 12.5┬▒3.3 MD: 12.5┬▒3.3 |
| Kim et al. [24] (2020) | Retrospective study | Korea | 30/30 | 64.23┬▒5.26/66.20┬▒6.01 | 13:17/12:18 | All single level | UBE: cerebrospinal fluid leak (n=1); MD: cerebrospinal fluid leak (n=2), surgical site infection (n=1) | VAS; ODI; operative time; dura expansion; estimated blood loss; serum creatine kinase; CRP; modified MacNab score; complication |
UBE: 12 MD: 12 |
| Heo et al. [25] (2018) | A case control prospective study | Korea | 46/42 | 65.8┬▒8.9/63.6┬▒10.5 | 18:28/16:26 | All single level | UBE: durotomy (n=1), postoperative hematoma (n=1); MD: durotomy (n=1), postoperative hematoma (n=2) | VAS for back and leg; ODI; operative time; cross-sectional area of the dura; complication |
UBE: 14.5┬▒2.3 MD: 14.5┬▒2.3 |
| Park et al. [26] (2020) | Randomized controlled trial | Korea | 32/32 | 66.2 (41ŌĆō80)/ 67.1 (45ŌĆō79) | 13 :19/18 :14 | All single level | UBE: incidental durotomy (n=2), postoperative epidural hematoma (n=1); MD: incidental durotomy (n=2), postoperative epidural hematoma (n=1), revision surgery due to recurrent pain (n=1) | VAS for back and leg; ODI; operative time; complication; EQ-5D score; hospital stay; postoperative drainage; serum creatine phosphokinase |
UBE: 12 MD: 12 |
| Kang et al. [27] (2019) | A prospective randomized comparative study | Korea | 32/30 | 65.1┬▒8.6/67.2┬▒9.5 | 18:14/14:16 | All single level | UBE: revision (n=1); MD: revision (n=1) | Operative time; hospital stay; Hemovac drain output; complication |
UBE: 6 MD: 6 |
| Choi et al. [28] (2019) | Retrospective study | Korea | 35/30 | 65.4┬▒11.8/65.2┬▒12.0 | 14:21/17:13 | UBE: single level (24), multiple levels (11); MD: single level (15), multiple level (15) | UBE: dural tear (n=2), root injury (n=1); MD: dural tear (n=2) | VAS for back and leg; ODI; CRP; complication; postoperative hemoglobin; transfusion |
UBE: 6 MD: 6 |
| Tuo et al. [29] (2021) | Retrospective analyzed | China | 22/25 | 59.1┬▒11.7/58.3┬▒8.7 | 12:10/11:14 | All single level | UBE: postoperative epidural hematoma (n=1), dura tear (n=3) MD: postoperative epidural hematoma (n=1), dura tear (n=5) | VAS; ODI; operative time; hospital stay; intraoperative blood loss; complication; modified MacNab criteria |
UBE: 12 MD: 12 |
| Lee et al. [30] (2020) | Retrospective | Korea | 59/44 | 69.64┬▒11.05/69.11┬▒8.58 | 30:29/16:28 | All single level | UBE: dura tear (n=6), infection (n=2), postoperative epidural hematoma (n=1), incomplete decompression (n=2), others (n=1); MD: dura tear (n=4), infection (n=2), postoperative epidural hematoma (n=1), others (n=1) | VAS for back and leg; ODI; operative time; drainage volume; hospital stay; complication |
UBE: 3 MD: 3 |
Table┬Ā2
| Study | Selection of participants | Confounding variables | Measurement of exposure | Blinding of outcome assessment | Incomplete outcome data | Selective reporting outcome |
|---|---|---|---|---|---|---|
| Min et al. [15] (2020) | Low risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
| Ito et al. [21] (2021) | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Kim et al. [22] (2018) | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Heo et al. [23] (2019) | Low risk | High risk | Unclear risk | High risk | Low risk | Low risk |
| Lee et al. [30] (2020) | Low risk | Unclear risk | Low risk | High risk | Low risk | Low risk |
| Choi et al. [28] (2019) | Low risk | High risk | Unclear risk | High risk | Low risk | Low risk |
| Tuo et al. [29] (2021) | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
| Kim et al. [24] (2020) | Low risk | High risk | Low risk | High risk | Low risk | Low risk |
| Heo et al. [25] (2018) | Low risk | Low risk | Low risk | High risk | Low risk | Low risk |
References
- TOOLS
-
METRICS

- Related articles in ASJ
-
Endoscopic Anterior Lumbar Interbody Fusion: Systematic Review and Meta-Analysis2023 December;17(6)






