Update of the Natural History, Pathophysiology, and Treatment Strategies of Degenerative Cervical Myelopathy: A Narrative Review
Article information
Abstract
Cervical myelopathy is a clinical syndrome resulting in symptoms of neurologic deficits due to prolonged spinal cord compression or ischemia in the cervical spine. Spinal cord compression can be caused by ossification of the posterior longitudinal ligament and hypertrophy of ligamentun flavum in addition to degenerative cervical spondylosis, degenerative disc disease, and progressive cervical kyphosis. Degenerative cervical myelopathy (DCM) is a series of disease entities caused by spinal cord compression by various non-traumatic and non-infectious causes. The pathophysiology of DCM includes spinal cord structure and function abnormalities caused by both static and dynamic factors. Surgical decompression for patients with moderate to severe cervical myelopathy not only inhibits the progression of neurological deterioration, but also improves functional status, pain, and quality of life. However, the role of nonsurgical treatment in patients with mild spinal cord compression is controversial. In general, patients with cervical myelopathies who do not undergo surgery have a poor prognosis. Appropriate surgical treatment is recommended when spinal cord compression is confirmed on image study in patients with reasonable symptoms of cervical myelopathy. The patient’s overall health, degree of compression, presence of concurrent cervical radiculopathy, and cervical spine alignment, in addition to lesion location and etiology, should be considered when determining an appropriate surgical procedure. This review covers the updated issues, including pathophysiology, clinical manifestations, differential diagnosis, and available treatments for DCM.
Introduction
Cervical myelopathy refers to a clinical syndrome caused by persistent cervical spinal cord compression or ischemia. This condition causes spinal cord dysfunction [1]. Various symptoms and signs may appear based on the anatomical location and the degree of spinal cord compression. Reduced agility and balance in the upper and lower legs may appear in the early stages; however, paralysis and incontinence may appear as spinal cord compression progresses. Patients with a sagittal diameter of the spinal canal of <12 mm have been demonstrated to be at risk for developing cervical myelopathy [2]. Absolute congenital canal stenosis is recognized if the canal diameter is <10 mm. Moreover, Pavlov et al. [3]. reported that the risk of cervical myelopathy increased when the spinal canal to vertebral body ratio was <0.82.
Cervical spondylosis, which is the degenerative change of the cervical spine, is the most common etiology of spinal cord compression, and it affects approximately 55% of patients with cervical myelopathy. The spinal canal continuously narrows to varying degrees due to a degenerative change of the disc. Additionally, osteophytes progression at the upper and lower endplates, the facet joint and/or uncinate process hypertrophy, and intervertebral disc herniation causes cervical myeloradiculopathy. The spinal canal may be further reduced by static factors, such as facet cysts and ossification of the posterior longitudinal ligament (OPLL), which may exacerbate cervical myelopathy.
The ligamentum flavum’s buckling could be a dynamic factor causing cervical myelopathy. Cervical cord compression may present as a dynamic compression if the sagittal diameter is <11 mm during maximal flexion-extension view. Cervical spine instability can be diagnosed by a translation of >3.5 mm or angulation of >11° in the dynamic view. The spinal cord undergoes extensive or localized axonal damage from increased strain or shear force in addition to mechanical or dynamic compression. Excessive cervical spine flexion can cause dynamic compression by stretching the spinal cord within the spinal canal. Compression and increased tension on the anterior spinal cord by excessive cervical flexion can exacerbate cervical myelopathy in the form of progressive cervical kyphosis.
Degenerative cervical myelopathy (DCM) is a collective term for neurological disorders caused by cervical spinal cord compression due to degenerative spondylosis, OPLL, and degenerative intervertebral disc herniation [4]. Patients with DCM are susceptible to acute spinal cord injury (SCI) following hyperextension injury falls or car accidents. It is mainly expressed as upper extremity numbness, gait disturbance, and weakness that worsen after hyperextension-type injury in elderly patients with degenerative spondylosis, and incomplete cord injury in the form of central cord syndrome is common [5]. Patients with DCM experienced higher hospitalization after SCI at rates of 13.9 and 4.8 per 1,000 person-years in Taiwan, respectively [6].
Recently, repetitive cord compression with cervical instability and/or spondylolisthesis has been proposed as a contributing factor in neurological deterioration in patients with DCM. Recent studies revealed poorer neurological baseline function and neurological outcomes after decompressive surgery in patients with DCM having spondylolisthesis. This patient group may have revealed a poorer prognosis than the general population due to the accumulation of repetitive minor trauma [7].
Ischemia is a significant pathologic factor that aggravates cervical myelopathy. Karadimas et al. [8] reported a reduced central gray matter perfusion through intramedullary branches when the anterior portion of the spinal cord was compressed, as well as reduced perfusion of the anterior sulcus artery through the transverse arterioles when the posterior portion of the spinal cord was compressed. Oligodendrocytes, which function to cover axons in myelin sheaths, are sensitive to ischemic injury and can undergo apoptosis following acute traumatic injury or long-term ischemic injury [9]. The concentration of extracellular glutamate is increased by a biochemical chain reaction in the spinal cord under ischemic conditions, and related cation-mediated cell damage results in apoptosis [10]. Ischemic injury frequently inhibits the electric conduction of the corticospinal tract. Recently, an apoptosis theory was developed to explain active spinal cord cell death following ischemic or traumatic injury [11]. Apoptosis occurring after acute SCI is known to cause secondary degeneration in lesions that are directly related to chronic demyelination in regions distant from the lesion [12]. Additionally, cervical spine stenosis causes ischemic changes and reduced circulation to the cord, thereby initiating a neurologic deterioration in patients with DCM [13]. Apoptosis of oligodendrocytes occurs before axonal degeneration in myelopathy, and this immediate apoptosis of oligodendrocytes can cause extensive and irreversible neurological deficits in a chronic state [14]. This review article covers the pathophysiology, clinical manifestations, differential diagnosis, and available treatments for DCM.
Clinical Symptoms and Signs
Clinical symptoms usually appear after the age of 40 years in patients with DCM, as degenerative changes progress, and are approximately 50% more common in males than in females [15]. Patients with DCM have a slowly deteriorating disease course and complain of general paresthesia of the upper extremities and clumsiness of the hands. Elderly patients may have a rapid DCM deterioration after hyperextension neck injury (Fig. 1) [16]. Some patients might not feel any pain or neurological dysfunction despite severe cord compression. Symptoms and signs of myelopathy may vary depending on the involved cervical segment and the degree of compression. Additionally, patients may exhibit gait disturbance, spasticity, and bladder or bowel dysfunction, along with increased cord compression severity [17].
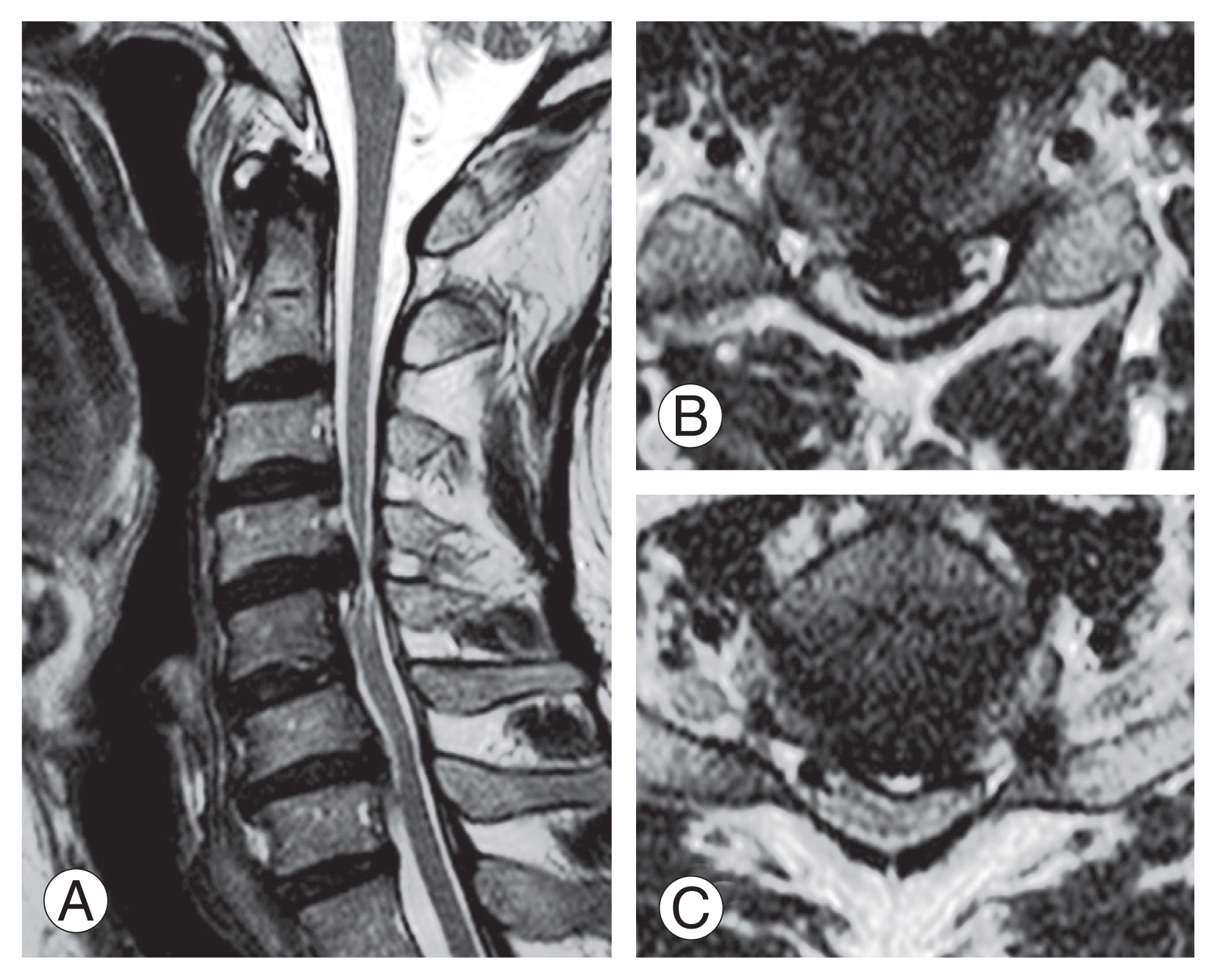
A 61-year-old male visited complaining of lower extremity muscle weakness, gait disturbance, and loss of dexterity in both hands that had worsened for 2 weeks ago. On the magnetic resonance image (A), cord signal changes were observed along with severe cord compression at C4/5 (B) and C6/7 (C) levels.
Patients with DCM may experience upper and lower extremity muscle weakness, paresthesias, urinary incontinence, and gait disturbance. Additionally, they complain of decreased fine motor skills such as chopsticks or handwriting. Hyper-positivity of the deep tendon reflex (DTR), ankle clonus, Babinski sign, and Hoffman sign may be seen as positive in patients with DCM [18]. Decreased pain and temperature sensation and position and vibration sensation can be seen on either side of the upper and/or lower extremities. However, DTR of the upper extremities may not increase when cervical radiculopathy and myelopathy coexist [19].
Diagnostic Tests
Simple radiography typically reveals disc space narrowing, sclerosis, osteophytes at the involved endplate, and uncovertebral and facet joint hypertrophy. Foraminal stenosis by uncinate process hypertrophy can be diagnosed in the oblique view. The lateral view is important for validating disc space narrowing, the osteophytes at the level-specific endplates, and the sagittal alignment. Longitudinal bony structures can be observed posterior to the vertebral body or intervertebral disc with OPLL. The execution of the swimmer’s view can be helpful when the endplate of T1 is invisible in the lateral view.
Magnetic resonance imaging (MRI) is the most important diagnostic tool for diagnosing patients with cervical myelopathic symptoms and signs. The degree of the degenerative changes of the intervertebral disc, cord compression, and hypertrophy of the ligamentum flavum can be observed on an MRI. Several studies revealed a correlation between signal changes in the spinal cord and the degree of cervical myelopathy [10–23]. Sharp, intense, multisegmented increased signal intensity in the spinal cord at T2-weighted images is correlated with suboptimal outcomes [21]. Additionally, improved functional outcomes are correlated with postoperative T2 signal intensity regression. In particular, Suri et al. [22] revealed that a combination of decreased signal intensity at T1 and increased signal intensity at the T2-weighted images resulted in poorer surgical outcomes compared to increased signal change at only the T2-weighted images [23]. Computed tomography (CT) may be a useful diagnostic tool for patients for whom MRI is contraindicated. The spinal axis can be visualized in three dimensions using CT and aids in the identification of bony anomalies and the ossification of ligamentous structures. CT scans can be used to diagnose the types and the degree of ossification (Fig. 2).
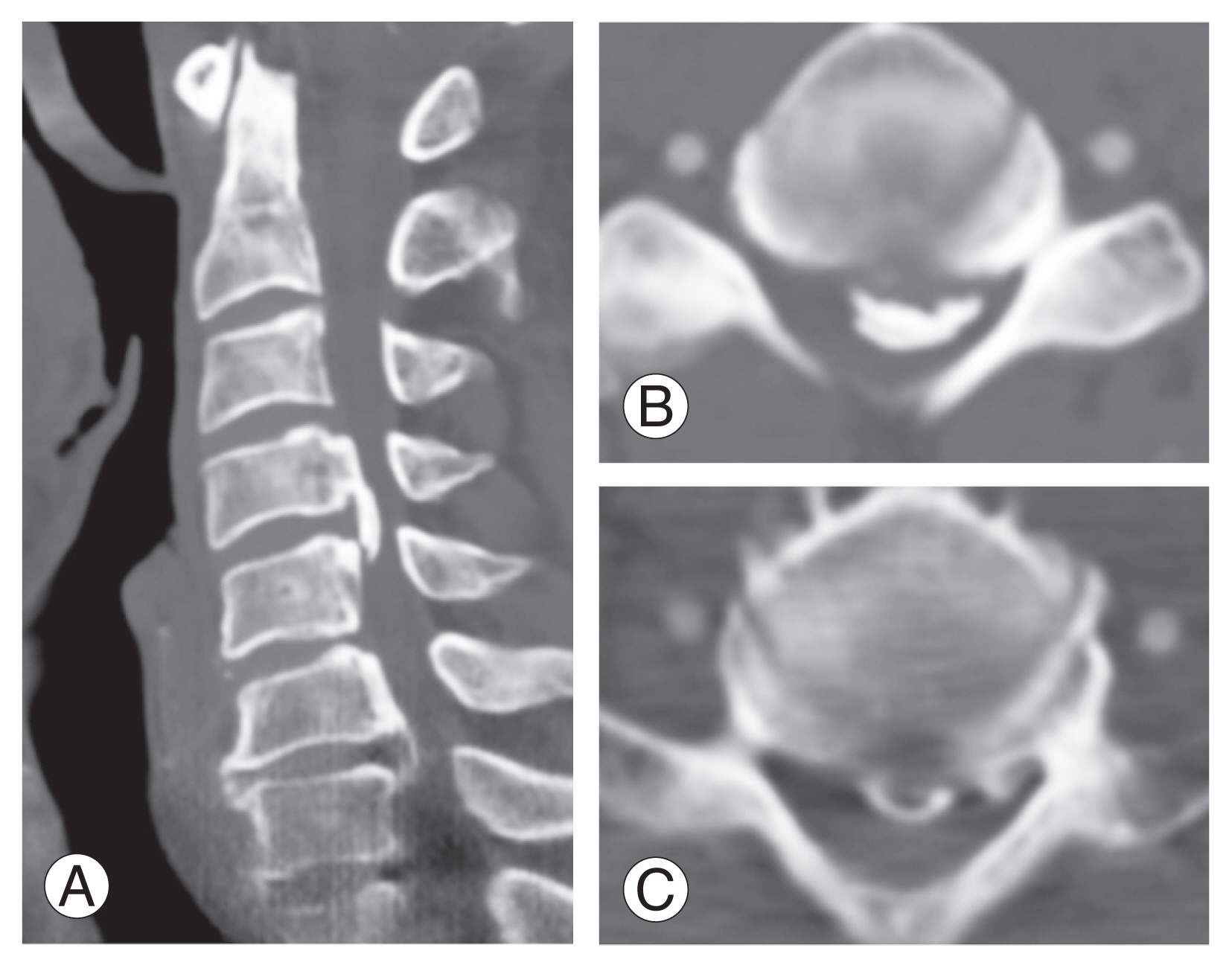
On computed tomography, ossification of the posterior longitudinal ligament was observed (A) at C4/5 (B) and C6/7 (C) levels, and severe compression of the spinal cord was observed along with loss of lordosis.
Electromyography (EMG) and nerve conduction studies are helpful to differentiate between cervical myelopathy and other neurological disorders. Electrophysiological examinations, including EMG, are not typically executed to diagnose patients with DCM. However, EMG can evaluate the spinal cord’s functional involvement and give additional clues when MRI findings are suspicious. Fasciculations, insertional activity increment, and decreased motor unit recruitment on needle EMG are signs of anterior horn cell damage or nerve root compression to assess the abnormalities of the motor unit [24]. Additionally, positive sharp waves, fibrillations, and abnormalities of spontaneous activity may manifest when the denervation is not completely compensated for. The severities of central conduction delay in patients with DCM can be determined using somatosensory evoked potentials (SEPs) via the tibial and median nerves. A baseline study would be needed preoperatively in cases of severe damage in amplitude and latency. Intraoperative spinal cord monitoring via evoked potentials is recommended to detect the greatest degree of conduction delay.
EMG studies can give immediate changes in muscle activation and recruitment in patients with DCM. EMG directly measures muscle activity; thus, it can be a useful tool for tracking disease progression, predicting surgical recovery, and assessing motor performance improvement after a rehabilitation trial. Injuries can occur anywhere along with the dorsal column-medial lemniscal pathway according to SEP [25]. This pathway is initiated by the stimulation of large myelinated afferent fibers in the peripheral nerves. The dorsal root ganglion, where the cell body is housed, receives information about proprioception, vibration, and delicate touch.
Motor-evoked potentials (MEPs) can detect damage to the descending motor pathways by the accumulation of transcranial stimulation of the motor cortex. Moreover, MEPs can assist in localizing the degrees of motor function abnormalities and identifying the involvement of descending pathways in patients with asymptomatic DCM. Additionally, MEPs help differentiate DCM from amyotrophic lateral sclerosis. Moreover, SEPs and MEPs may help predict disease progression in patients with cervical cord compression on imaging studies but without myelopathy symptoms. Generally, myelopathy is more likely to occur if abnormal MEP and SEP are observed in non-myelopathic patients who have spinal cord compression. Additionally, MEPs and SEPs help identify neurological injury and recovery following surgery.
Natural History
Aging is the main cause of degenerative spondylosis and disc degeneration, and the average age of onset is usually between the mid-50s and 60s. Additionally, >60% of patients are males, suggesting that males have a higher risk of developing DCM. Incidence differed by gender (28.9 males versus 15.3 females per 100,000 person-years), with the highest incidence of DCM in both males and females in their 70s. DCM in males was characterized by more severe degenerative changes, multilevel spinal cord compression, and high-frequency T2 hyperintensity changes on MRI [26].
The prevalence of non-myelopathic spinal cord compression (NMSCC) was reported in 64 (5.3%) of 1,211 patients who underwent MRI in Japan [27]. Additionally, elderly patients have a higher prevalence of spinal cord compression without clinical symptoms of myelopathy. The ratio of NMSCC was 57.9% of the 183 volunteers over the age of 40 years who underwent cervical MRI [5,28]. Furthermore, these patients with NMSCC had macro and micro-structural changes in the spinal cord comparable to patients with symptomatic DCM based on recent imaging studies [26]. A recent meta-analysis reported the prevalence of NMSCC in the different study demographics as 24.2% in the healthy population and 35.3% in the population aged 60 years or older [29].
The clinical importance of NMSCC is that patients are likely to progress eventually. Approximately 10% of the 20 patients with NMSCC eventually experienced myelopathy symptoms after a mean follow-up duration of 21 months. Bednarik et al. [30] revealed that symptoms of myelopathy can develop in 8% of patients at a 1-year follow-up and approximately 20% at a 4-year follow-up. Patients with cervical neuropathy, abnormalities in somatosensory and MEPs, and high signal intensity on T2-weighted images on MRI are reported to predict the development of myelopathy at 1-year follow-up [23,30]. However, the importance of T2 signal changes alone is unclear in determining the likelihood of cervical myelopathy progression. A multivariate analysis study predicted DCM to occur in the presence of radiculopathy, an axial area of <70.1 mm2, and a compression ratio of >0.4 [31]. Abnormal findings in SEPs and MEPs have been associated with the development of cervical myelopathy, and these findings have been suggested as indications for recommending surgical treatment in patients with NMSCC.
Nonoperative Treatments
Options for nonoperative treatment of DCM include cervical traction, bracing, analgesics, therapeutic exercise, manual therapy, bed rest, and avoiding hazardous situations and physical activities such as contact sports. However, the evidence for the role of nonsurgical treatment in patients with DCM remains lacking. Several studies in patients with DCM undergoing nonoperative treatment revealed very low effectiveness, and changes in modified Japanese Orthopedic Association (mJOA) scores usually ranged from 0 to 1. Only one of eight eligible studies in a thorough systematic review published in 2017 revealed functional improvements that were greater than the reported minimal difference in mJOA score that was clinically significant [32]. The literature revealed the limited effectiveness of nonsurgical treatment, as 23%–54% of patients receiving nonsurgical management eventually require surgical treatment within an average follow-up of 29–74 months [18]. Therefore, even patients with asymptomatic myelopathy are important to be referred to a spine specialist for appropriate management in the future.
Operative Treatments
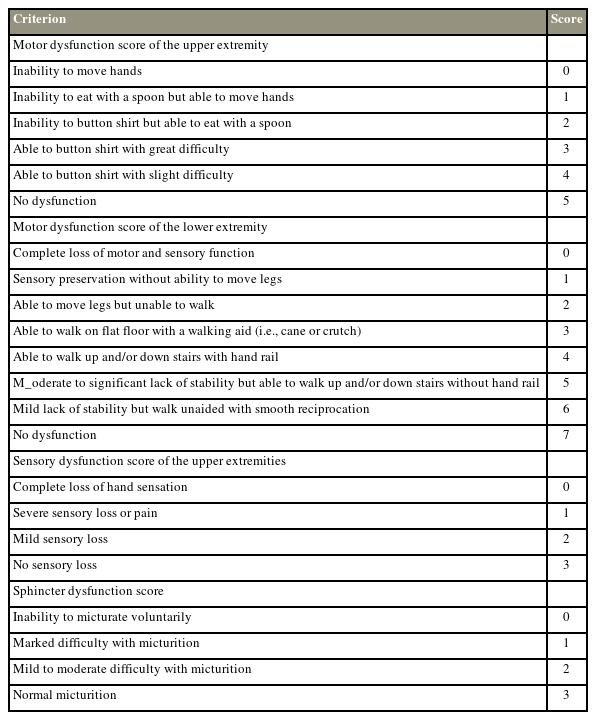
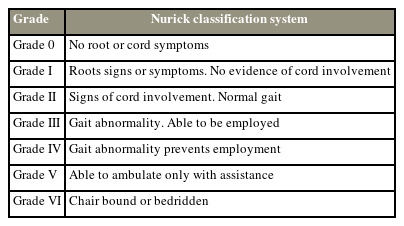
A prospective multicenter study reported that surgical decompression could decelerate the progression of myelopathy, as well as improve patient-reported outcomes (PROs), neurological status, and quality of life. They reported improvements in mJOA, Nurick grades, and PROs at 1-year follow-up in 85.4% of patients who underwent surgical decompression (Tables 1, 2) [33].
Many factors, such as the cause of the disease, the alignment of the spine in addition to the main segments that cause myelopathy, and the presence or absence of tandem stenosis should be considered, to determine the optimal surgical method for patients with cervical myelopathy. Anterior surgery is commonly performed in short-segment herniated discs and cervical spondylosis and is recommended in situations where the major pathology is anteriorly located. Additionally, vertebral body sliding osteotomy (VBSO) can be performed for patients with severe OPLL for whom anterior cervical discectomy and fusion (ACDF) or anterior cervical corpectomy and fusion are not feasible [34]. Favorable VBSO results have been reported for sufficient decompression, recovery of cervical alignment, and high fusion rate not only in OPLL but also in patients with CSM [35]. Additionally, simultaneous anterior-posterior corrective surgery can be performed for safe and efficient spinal decompression in the case of multiple cervical spondyloses and OPLL with multi-segmental spinal cord compression along with cervical kyphosis [36] (Fig. 3).
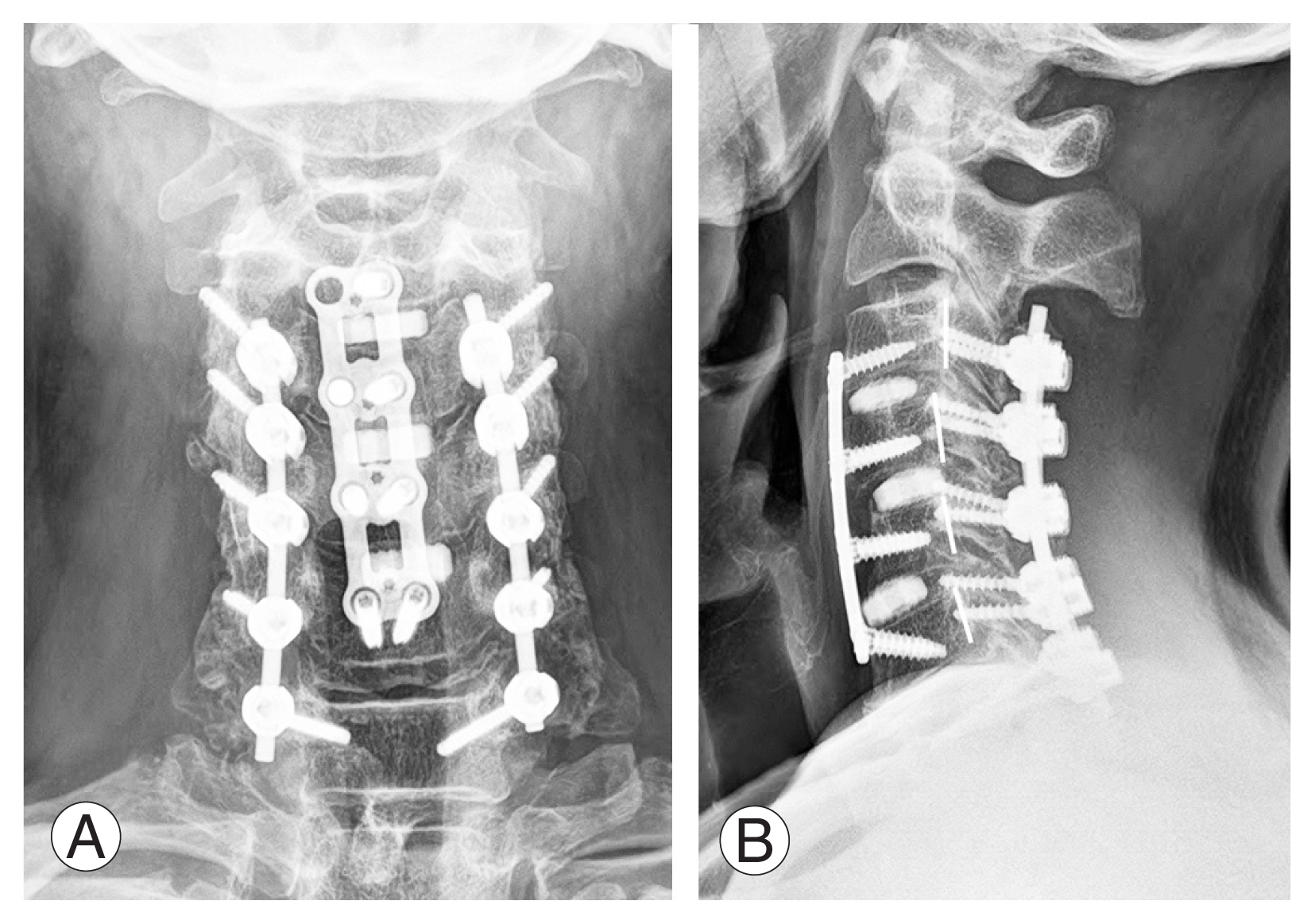
For recovery of cervical lordosis and adequate decompression of the spinal cord, posterior instrumentation with laminectomy was followed by anterior vertebral body sliding osteotomy at C4 and C5, and finally posterior fusion with the autologous bone graft from C3 to C7 was performed (A, B).
1. Anterior surgery
Smith and Robinson [37] and Cloward [38] developed the anterior cervical approach in the 1950s. Anterior cervical spine surgery approaches the interfacial plane in the supine position with the neck slightly extended. Trachea, esophagus, and recurrent laryngeal nerves are retracted medially, and the carotid sheaths are retracted laterally. The anterior approach can directly remove the intervertebral disc, osteophyte, and OPLL that compress the spinal cord; thus, it is advantageous for the main pathologies located anteriorly. Arthrodesis or arthroplasty is additionally needed after intervertebral disc decompression. The final fusion rate is reported to be approximately 95% in the case of single-segment fusion. Patients who additionally performed plate fixation demonstrated a high spinal fusion rate and a low subsidence rate and better neck pain scores [39]. Additionally, additional uncinate resection performed simultaneously during ACDF was reported to not affect the fusion rate in patients with concomitant myeloradiculopathy [40].
Dysphagia is one of the most frequent unintended consequences after anterior surgery. High intratracheal pressure or longer duration of surgical time affect postoperative dysphagia. Postoperative dysphagia occurs relatively commonly, ranging from 2% to 48%; however, many patients improve spontaneously within 3 months postoperatively. Conversely, the incidence of esophageal perforation after anterior cervical spine surgery is very low, ranging from 0.2% to 0.9% [41]. The multifactorial causes of postoperative dysphagia are related to the hematomas postoperatively, and upper esophageal denervation is caused by pharyngeal plexus injury or excessive traction. Temporary hoarseness affects 3%–11% of patients, whereas constant hoarseness happens in 0.33% of patients [42]. Postoperative dysphonia shows a high frequency in reoperation and is closely related to damage to the recurrent laryngeal nerve or superior laryngeal nerve. The incidence of postoperative dysphagia was significantly reduced when the pressure of the endotracheal cuff was lowered to 20 mm Hg after anterior cervical surgery [43]. Postoperative airway obstruction due to edema or hematoma formation is the most fatal complication after anterior cervical spine surgery and requires careful attention. Patients undergoing upper cervical surgery, severe myelopathy, a protracted surgical period, and a difficult airway should be considered to be kept intubated postoperatively until 48 hours to avoid postoperative airway complications [44].
2. Posterior surgery
A laminectomy or laminoplasty with a posterior approach effectively decompresses the spinal cord in patients with DCM having multilevel compression. The posterior laminectomy was developed in 1901 as a treatment for patients with cervical fractures and later used as a treatment for patients with multilevel cervical stenosis. However, some authors have reported that neurological symptoms may be aggravated by post-laminectomy kyphosis and scar tissue development around the dura after laminectomy. Additionally, post-laminectomy syndromes, such as segmental instability and kyphosis, have been documented [45]. Zdeblick and Ducker [46] reported cervical instability when >50% of the facet joint is removed during a foraminotomy. Further, through biomechanical studies, Nowinski et al. [47] reported that a facetectomy of >25% might affect the cervical spine’s stability following multilevel laminectomy. Therefore, additional instrumentation and fusion are needed to avoid iatrogenic instability and kyphosis after laminectomy. The most common method for rigid internal fixation is lateral mass screw instrumentation and autologous bone fusion. However, pseudarthrosis, loosening, adjacent segment disease, and progression of kyphosis are drawbacks of laminectomy and fusion [48].
Laminoplasty was introduced in Japan in 1973 as a new procedure that can sufficiently decompress the spinal canal while maintaining the posterior facet joint [49]. Two types of laminoplasty can be performed; one is open-door laminoplasty, which widens the spinal canal by creating a single-side hinge between the lamina and facet joint, and the other is double-door laminoplasty, which creates a two-side hinge and opens the center of the spinous process [50]. A keyhole foraminotomy to treat the accompanying radiculopathy can be performed before expanding the posterior arch. Chiba et al. [51] reported a significantly improved average JOA score and recovery rate for up to 3 years postoperatively and slightly decreased at 5 years postoperatively, but an acceptable range was maintained in 80 patients who underwent open-door laminoplasty. The mean JOA score and recovery rate after laminoplasty significantly improved postoperatively; however, the late deterioration post-laminoplasty kyphosis, OPLL progression, and the decrease of range of motion are reported [52].
Conclusions
Cervical myelopathy may manifest various types of symptoms and signs as degenerative spondylosis and intervertebral disc degeneration progress. Appropriate surgical treatment is recommended when spinal cord compression is confirmed on MRI in patients with reasonable symptoms of cervical myelopathy. Adequate surgical decompression can prevent disease progression as well as improve pain and quality of life. The patient’s overall health, degree of compression, presence of concurrent cervical radiculopathy, and cervical spine alignment, in addition to the location of the lesion and the etiology, should be considered when determining an appropriate surgical procedure.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: CNK; data curation: MWK; formal analysis: MWK; project administration: SHC; writing-original draft: SHC; writing-review & editing: MWK; and final approval of the manuscript: all authors.