|
 |
- Search
| Asian Spine J > Volume 17(4); 2023 > Article |
|
Abstract
The perioperative management of patients medicated with antithrombotic agents who require elective spinal surgery is extremely challenging because of the increased risk of surgical bleeding and the concurrent need to minimize the thromboembolic risk. The aims of the present systematic review are to: (1) identify clinical practice guidelines (CPGs) and recommendations (CPRs) on this topic and (2) assess their methodological quality and reporting clarity. An electronic systematic search of the English Medical Literature up to January 31, 2021 was conducted using PubMed, Google Scholar, and Scopus. Two raters assessed the methodological quality and reporting clarity of the gathered CPGs and CPRs using the Appraisal of Guidelines for Research and Evaluation II (AGREE II) tool. The agreement between the two raters was assessed using Cohen’s kappa. Of the initially gathered 38 CPGs and CPRs, 16 fulfilled our eligibility criteria and were evaluated using the AGREE II instrument. The reports published by “Narouze 2018” and “Fleisher 2014” were scored as being of “high-quality” and having an adequate interrater agreement (Cohen’s kappa ≥0.60). Overall, the AGREE II domains of “clarity of presentation” and “scope and purpose” yielded the highest scores (100%), whereas the domain “stakeholder involvement” scored the lowest score (48.5%). The perioperative management of antiplatelet and anticoagulant agents in elective spine surgery may be challenging. Because of the lack of high-quality data in this field, uncertainty remains regarding the optimal practices to balance the risk of thromboembolism against that of bleeding.
As the population ages, the number of patients receiving long-term treatment with antiplatelets or anticoagulants continues to rise [1,2]. Antiplatelet agents constitute an essential pillar of the management of both coronary artery and peripheral vascular disease, whereas anticoagulants are the cornerstone of stroke prevention in relation to atrial fibrillation [1–4]. It has been reported that 250,000 patients on antithrombotic agents undergo elective surgery annually in North America [3,5–7].
Conversely, neurosurgical patients and those undergoing spinal surgery are regarded as having a “high bleeding risk,” as even small hemorrhages in closed spaces, such as the cranial vault and/or the spinal canal, can lead to harmful neurological consequences [3,8,9]. Therefore, the optimal perioperative management of these patients dictates that the bleeding risk should be balanced against the risk of thromboembolism. Both patient-related/procedure-specific bleeding and thromboembolic risk factors should be assessed, stratified, and properly adjusted in this context [1,3,5,6,8].
Although it is becoming clear that antiplatelet or anticoagulant therapies should be included among the aforementioned factors mentioned, their perioperative management remains challenging. First, the management of these patients requires the cooperation of various medical disciplines with diverse backgrounds and preferences [6,10–12]. Second, the perioperative management of these patients is guided based on recommendations that stem mostly from the opinions of experts. These recommendations lack specificity, and are frequently of poor methodological quality and reporting clarity [3–5,13].
To the best of our knowledge, the available recommendations on the perioperative management of patients receiving antithrombotic (antiplatelet or anticoagulant) agents undergoing elective spinal procedures have not been systematically reviewed and methodologically assessed. The aims of this systematic review are as follows: (1) to identify clinical practice guidelines (CPGs) and clinical practice recommendations (CPRs) regarding the management of antithrombotic (antiplatelet or anticoagulant) agents in elective spinal procedures and (2) to report and assess the reporting clarity and methodological quality of the relevant CPGs and CPRs.
We performed an electronic literature search for published CPGs or CPRs in PubMed, Google Scholar, and Scopus using the strategy depicted in Table 1. Additional CPGs were extracted from the reference list of the gathered records. In the current review, we focused on CPGs reporting on the perioperative management of patients on antithrombotic agents undergoing elective spinal procedures. We excluded CPGs that did not comment on the use of antithrombotic agents; however, we included general CPGs on the periprocedural use of antithrombotic agents, even if they did not focus solely on elective spinal procedures, according to Appraisal of Guidelines for Research and Evaluation II (AGREE II) [14,15].
Each CPG or CPR was identified by the name of the first author and the year of publication. For study selection, two authors assessed the titles and abstracts to eliminate records based on study design. Additional CPGs were discarded after reading the full text document. For the appraisal process, the full text of the gathered recommendations and their updated versions, including their supplements, were studied thoroughly. Two review authors with a high level of expertise in the perioperative management of antithrombotic therapy and spine surgery conducted the review process individually. The first author is an anaesthesiologist with more than 7 years of experience who (1) has a Master’s degree in thrombosis and antithrombotic treatment and is an invited lecturer on this subject, (2) is a member of a working group regarding thrombosis and antithrombotic therapy, and (3) is the author of the recently published guidelines regarding the perioperative management of antiplatelet therapy; and the second author is a senior and experienced spine surgeon who is also experienced in Bioinformatics and Biostatistics. Both review authors completed the AGREE II online training [6,14,15]. Additionally,, any upcoming issues which arose were discussed with a senior reviewer who is (1) the codirector of and a lecturer of a master’s degree on thrombosis and antithrombotic treatment; (2) a member of a scientific committee on transfusion, haemostasis, and thrombosis; (3) a member of a working group on thrombosis and antithrombotic therapy, and (4) the author of the recently published guidelines regarding the perioperative management of antiplatelet therapy. None of them had participated in the writing or development of any gathered records.
The AGREE II tool consists of 23 items organized in six domains (scope and purpose, stakeholder involvement, rigor of development, clarity of presentation, applicability, and editorial independence) and two additional items (overall assessment). Each item was rated on a 7-point Likert scale (1, strongly disagree to 7, strongly agree). The final domain scores were calculated according to the AGREE II tutorial and sample test practice guidelines. The two review authors rated each domain independently [14,15]. The results were then visualized in stacked bar plots. At present, no specific quality thresholds exist for classifying guidelines as having a high or low quality [16,17]. The domain scores were categorized as “high” (≥80%), “medium” (60%–79%), “low” (40%–59%), and “very low” (≤40%). The degree of agreement between the raters was determined based on weighted Cohen’s kappa; a value between 0 and 0.20 corresponded to “no agreement,” between 0.21 and 0.39 to “minimal agreement,” between 0.40 and 0.59 to “weak agreement,” between 0.60 and 0.79 to “moderate agreement,” between 0.80 and 0.90 to “strong agreement,” and above 0.90 to “almost perfect agreement.”
In anticipation of a deviation from the normal distribution, the results were summarized using median values together with their interquartile range and visualized in bar plots and box plots, respectively. Comparisons of mean group values were performed using Kruskal-Wallis nonparametric analysis of variance tests followed by Dwass-Steel-Critchlow-Fligner pairwise comparisons. All statistical analyses were carried out using Excel (Microsoft Corp., Redmond, WA, USA), the R statistical environment (https://www.r-project.org/), and the Real Statistics package (https://real-statistics.com/free-download/real-statistics-resource-pack/) [16]. Statistical significance was set at p<0.05.
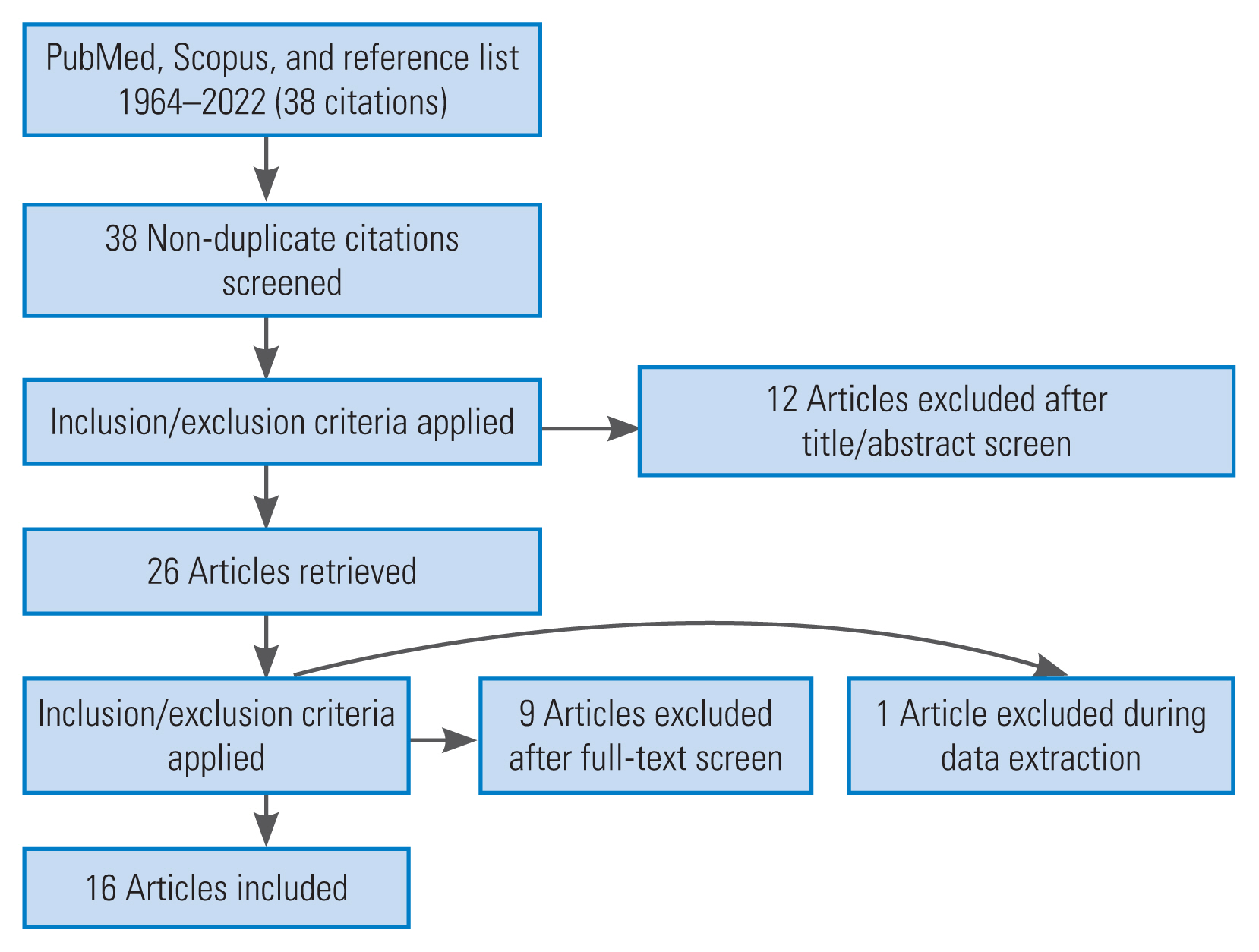
The literature search resulted in 38 unique CPGs and CPRs. Among them, 12 records were discarded as being irrelevant after reading the corresponding manuscript and abstract. For the same reason, 10 additional records were excluded after reading the full text. The remaining 16 CPGs formed the basis of our review (Fig. 1) [3–5,8,10,17–27].
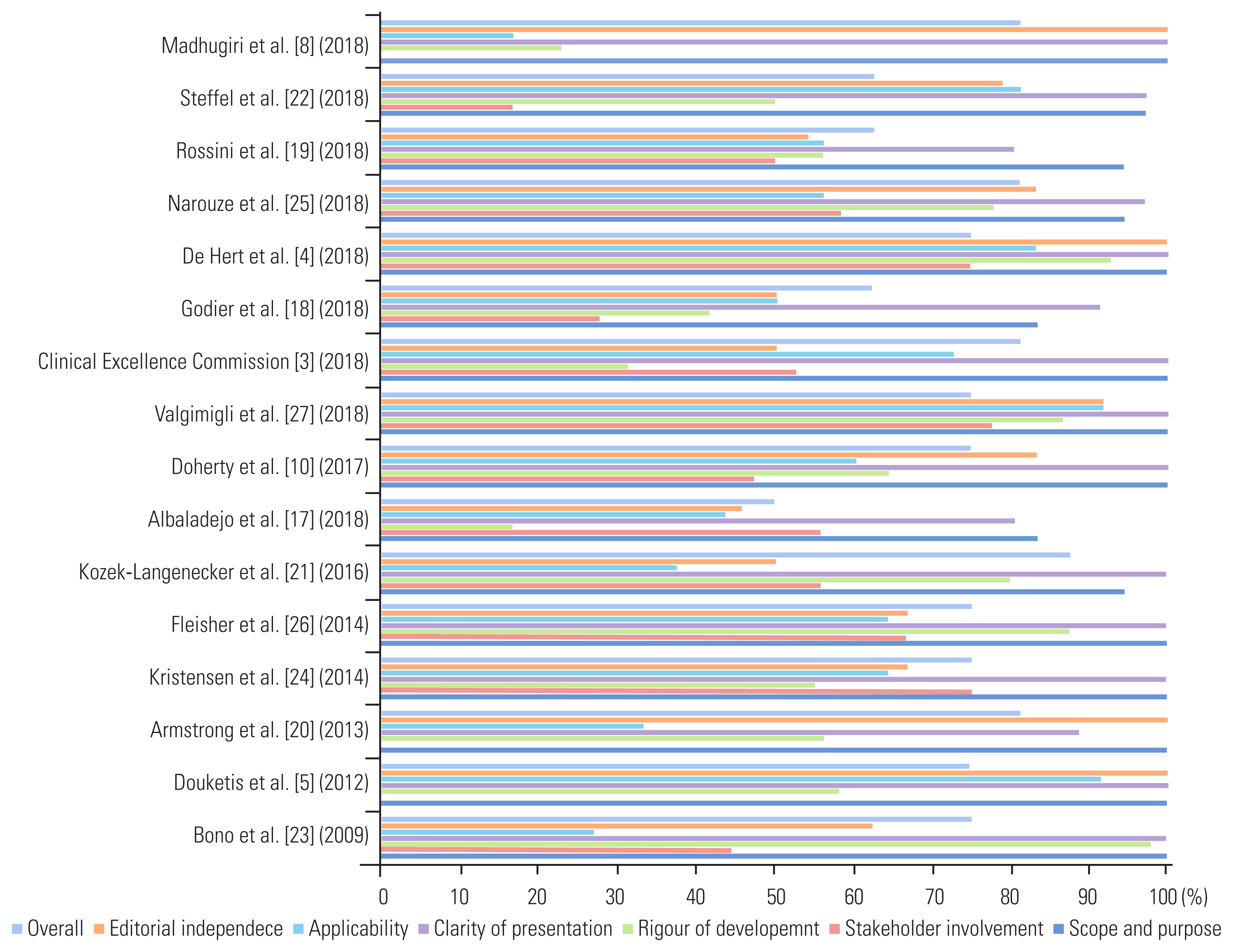
As depicted in Table 2, the top-5 ranking items included the CPGs reported by Kozek-Langenecker et al. [21] (88%), Narouze et al. [25] (81%), Clinical Excellence Commission [3] (81%), Armstrong et al. [20] (81%), and Madhugiri et al. [8] (81%). The CPS with the lowest scores were those reported by Albaladejo et al. [17] (50%), Godier et al. [18] (63%), Rossini et al. [19] (63%), and Steffel et al. [22] (63%) (Fig. 2).
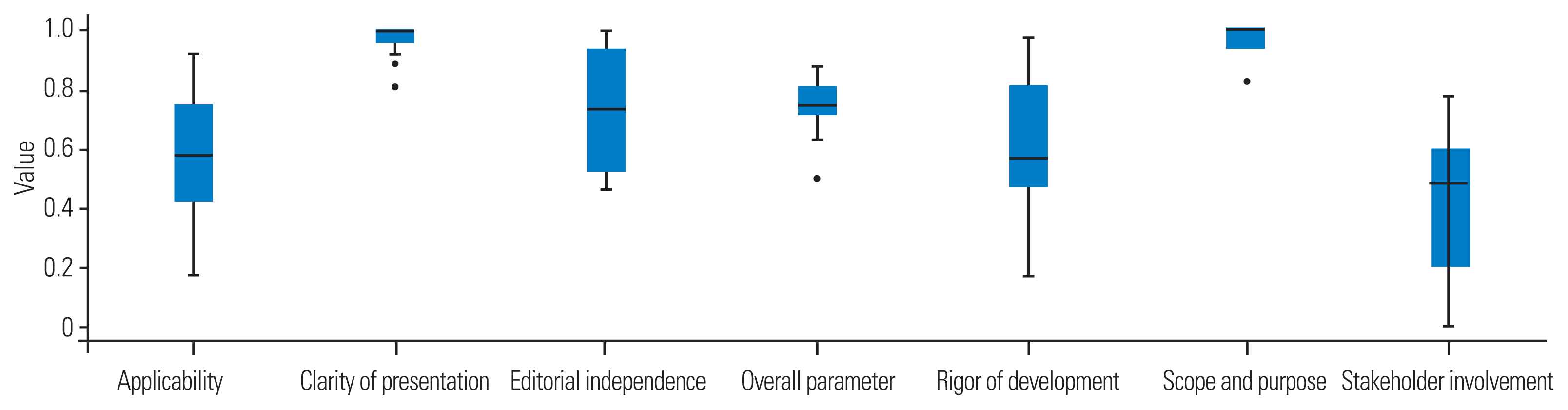
The highest scores were recorded for the “clarity of presentation” and “scope and purpose” domains (Table 3), reaching as high as 100%. In contrast, the lowest scores were observed for the “stakeholder involvement,” “rigor of development,” and “applicability” domains, with an average of 48.5%, 57%, and 58%, respectively (Fig. 3). The differences between the domain scores were frequently significant, particularly when comparing the high- and low-score domains (Table 4).
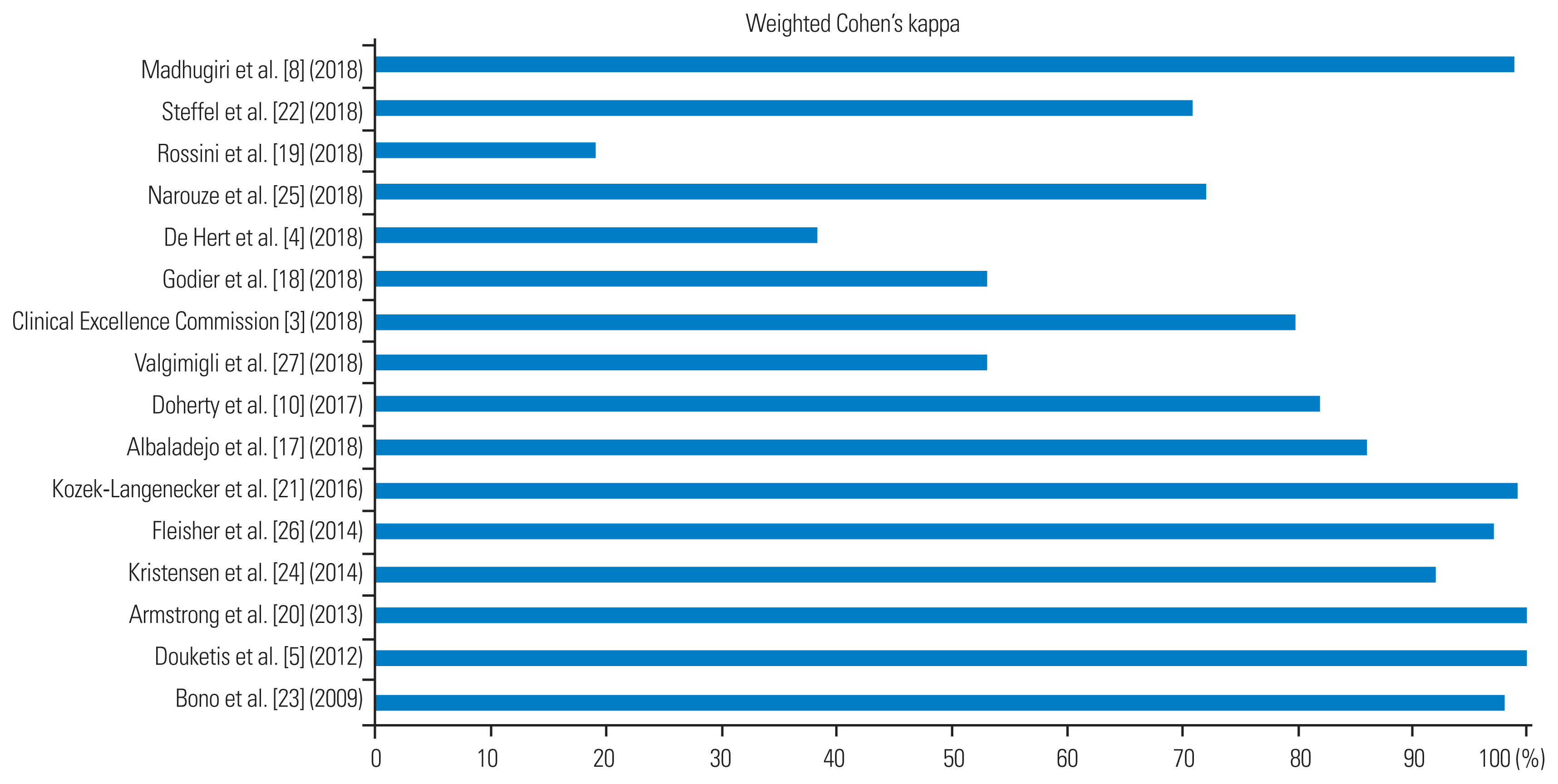
The mean interobserver agreement was as high as 84% (interquartile range, 66.5%–98.2%), corresponding to a “strong agreement” between the two reviewers (Table 5). However, large variations were noted among the scores of the individual CPGs, which ranged from as low as 19% for the CPGs reported by Rossini et al. [19] to as high as 100% for the CPGs reported by Douketis et al. [5] and Armstrong et al. [20] (Fig. 4).
The current systematic review revealed that the perioperative management of patients on antiplatelets or antithrombotic agents undergoing elective spinal procedures is guided by a plethora of CPGs. In addition to differences in the issuing body and the associated medical disciplines, there was substantial heterogeneity regarding the methodological quality and reporting clarity of these recommendations. The major limitations of the currently available CPGs were the underreporting of the CPG methodology, stakeholder involvement, and the absence of applicability tips and tricks. Regarding the methodological quality of CPGs and CPRs, several critical points need to be highlighted. The methodological framework of the eligible guidelines varied significantly. Hence, the processes linking the available evidence to the provided recommendations were frequently unclear. Furthermore, we noted substantial inconsistency in the scales used to assess the level of the available evidence and the strength of the recommendations among the CPGs. Consequently, the interpretation of the statements and the direct comparison of the guidelines based on their strength should be approached with extreme caution. To highlight the importance of the developmental process, the AGREE II tool requires “high-quality” CPGs or CPRs, with a score as high as 70% for the “rigor of development” domain, compared with 50% for the remaining five domains [14,15,28].
Among the available guidelines, two sets of CPGs fulfilled the criteria for a higher quality and had a high interrater agreement and are presented below. The 2014 American College of Cardiology/American Heart Association guideline reported on the perioperative cardiovascular evaluation and management of patients on antiplatelet mono or dual therapy undergoing elective noncardiac surgery [26]. The authors address the importance of the continuation of antiplatelet therapy when the risk of increased cardiac events outweighs the risk of increased bleeding. The value of the multidisciplinary approach and the teamwork among neurosurgeons, anaesthesiologists, and cardiologists for weighing the relative risk of bleeding against that of thrombosis was also emphasized [26]. Finally, the consensus between the treating physician and the patient regarding the perioperative plan was also highlighted [25]. However, the recommendations were mostly based on a consensus based on the opinions of experts, small or retrospective studies, and registries, whereas the strength of the recommendations was rated “level of evidence C” [26]. Of note, the guideline panel consisted of 17 physicians, mainly cardiovascular specialists. The conflicts of interest of all panel members within 5 years of the publication of the guidelines were listed.
Furthermore, with respect to patients undergoing interventional spine and pain procedures, the guidelines provided by the American Society of Regional Anesthesia and Pain focus on antiplatelet and/or anticoagulant agents [25]. The guideline panel consisted of 124 physicians; 84% of the respondents were anaesthesiologists, and the remainder were neurological surgeons, orthopedic surgeons, neurologists, physical medicine specialists, and rehabilitation physicians. The issuing group acknowledged the challenge of the classification of interventional procedures for spine pain with respect to the potential risk of bleeding complications and patient-related bleeding factors. Ultimately, they recommended that patients with a high risk of bleeding (e.g., old age, history of bleeding tendency, concurrent use of other antithrombotic agents, liver cirrhosis or advanced liver diseases, and advanced renal disease) who undergo low- and/or intermediate-bleeding risk procedures should be treated as having an “intermediate” or a “high” bleeding risk [25]. Notably, the authors appreciated the lack of high-quality data regarding the perioperative management of procedures with a high bleeding risk in patients treated with antithrombotic agents. Therefore, they recognized that it was not possible to assess the recommendation strength or grade because of the lack of data from randomized studies or from those including large numbers of patients from registries. However, they suggested that their CPGs could provide sound recommendations as well as evidentiary basis for such recommendations, as they were extracted based on the current best available quality data [25].
Based on the CPGs with the highest quality and the highest interrater agreement [25,26], a classification of the potential risk of serious bleeding in elective spinal procedures (Table 6) was proposed. Based on this classification, Tables 7 and 8 provides a summary of the proposed recommendations for the periprocedural management of antithrombotic agents in the elective spinal surgery setting.
It should be highlighted that patients with a patient-related risk of bleeding, i.e., advanced age, bleeding tendency, concurrent use of other antithrombotic agents, and advanced liver or renal disease, who present for low-risk procedures should be treated as undergoing intermediate-risk procedures. In patients treated with low-dose aspirin (81–100 mg)/aspirin combinations undergoing low-bleeding procedures (Table 6), the antiplatelet agent could be continued perioperatively (Table 8). Regarding procedures with a high or intermediate risk of bleeding in patients who are treated with aspirin/aspirin combinations for secondary prophylaxis and who are characterized as having a high thrombotic risk, shared assessment and risk stratification are proposed (Table 8). Similarly, in patients treated with phosphodiesterase inhibitors and non-aspirin nonsteroidal anti-inflammatory drugs, no discontinuation of the antithrombotic agent is proposed in procedures with a low risk of bleeding (Table 8). In patients treated with anticoagulants, such as vitamin K antagonists and direct oral anticoagulants, continuation of the anticoagulant agent could be proposed in procedures with a low risk of bleeding based on shared assessment and risk stratification (Table 8).
The current systematic review had several important limitations. In anticipation of a limited number of guidelines on elective spinal procedures, we used a more inclusive approach and a more exhaustive literature search. Therefore, our review was based on heterogeneous CPGs and CPRs focusing on surgical patients in general. Concomitantly, our review exhibited significant variation in the interrater agreement among the eligible items. However, there is no gold-standard procedure for estimating the interrater agreement because both percent agreement and Cohen’s kappa have important limitations. The weighted Cohen’s kappa metric was designed to exclude the possibility that the raters guessed the scores; thus, it may lower the estimate of the agreement excessively as the assumptions it makes are not well supported [29]. Nevertheless, experts have suggested that little confidence should be placed in the results for any study with a kappa value below 0.60, as this indicates inadequate agreement among the experts [29]. In turn, the different backgrounds of the review authors (anesthesia and neurosurgery) may strengthen the results in terms of a more multidisciplinary approach. Of equal importance, the AGREE II tool also has several inherent limitations. Over the years, it has received criticism as it relies on subjective assessments, resulting in a limited interrater agreement. Similarly, the domains are not weighted, and, because of the lack of scores with a clear cutoff value, it may prove quite challenging for the review author to reach unbiased conclusions regarding the true quality of the assessed CPGs and CPRs [2].
The current systematic review emphasized on the lack of high-quality data in the field of the perioperative management of antithrombotic agents in elective spine surgery and the urgent need for the development of recommendations based on high-quality primary studies in the field of the perioperative management of antiplatelet and anticoagulant agents in elective spine surgery, as there great uncertainty remains regarding the optimal practices for balancing the risk of thromboembolism against that of bleeding. Moreover, it seems that, as most neurosurgical procedures are classified as having a high and intermediate bleeding risk and because even small hemorrhages in closed spaces (such as the spinal canal) may lead to harmful neurological consequences, the majority of the decisions are based on a more conservative approach regarding the continuation of the antithrombotic agents. Hence, the decision to discontinue the antithrombotic agents is based more often on the fear of bleeding, thus exposing the patients to a risk of thrombosis, in whom acute myocardial infarction, acute stroke, and so forth may result in life-threatening complications [3,8,9]. Therefore, there is an imperative need to develop level-I evidence based on well-designed studies and to form methodologically sound guidelines with a rigorous, balanced, and evidence-based process that involves all relevant stakeholders.
The “Perioperative Anticoagulation Use for Surgery Evaluation study” could serve as an example of the methodological development of additional studies regarding the perioperative management of antiplatelet and anticoagulant agents in settings with a high bleeding risk, whereas it seems that the results of such studies could be used to develop methodologically sound guidelines with level-I evidence for patients undergoing elective spinal procedures who are treated with antithrombotic agents [30].
The perioperative management of antiplatelet and anticoagulant agents in elective spinal surgery remains challenging. Due to the lack of high-quality data in this field, uncertainty remains regarding the optimal practices for balancing the risk of thromboembolism against that of bleeding. Therefore, there is an imperative need to develop level-I evidence and to form methodologically sound guidelines with a rigorous, balanced, and evidence-based process that involves all relevant stakeholders. Finally, decisions regarding the complicated and challenging perioperative management of antithrombotic agents should be based on a multidisciplinary and patient-centered approach executed by a team of experts using the guidelines available currently.
Notes
Author Contributions
Conceptualization: MPN, AGB, FAA, MP, MM, KNF, EMA; methodology: MPN, AGB, FAA, MP, MM, KNF, EMA; data curation, Formal analysis: AGB; investigation: MPN, AGB, FAA, MP; project administration: MPN, AGB, FAA, MP, MM, KNF, EMA; resources: MPN, AGB, FAA, MP; validation: MPN, AGB, FAA, MP, MM, KNF, EMA; writing–original draft: MPN, AGB, MM, KNF, EMA; writing–review & editing: MPN, AGB, FAA, MP, MM, KNF, EMA; and supervision: MM, KNF, EMA.
Table 1
Search strategy of the current review
Table 2
Individual Appraisal of Guidelines for Research and Evaluation II score of guidelines for the eligible clinical practice guidelines
| Recommendations | Scope and purpose | Stakeholder involvement | Rigor of development | Clarity of presentation | Applicability | Editorial independence | Overall | Average |
|---|---|---|---|---|---|---|---|---|
| Albaladejo et al. [17] (2018) | 0.83 | 0.56 | 0.17 | 0.81 | 0.44 | 0.46 | 0.50 | 0.54 |
| Godier et al. [18] (2018) | 0.83 | 0.28 | 0.42 | 0.92 | 0.50 | 0.50 | 0.63 | 0.58 |
| Madhugiri et al. [8] (2018) | 1.00 | 0.00 | 0.23 | 1.00 | 0.17 | 1.00 | 0.81 | 0.60 |
| Rossini et al. [19] (2018) | 0.94 | 0.50 | 0.56 | 0.81 | 0.56 | 0.54 | 0.63 | 0.65 |
| Armstrong et al. [20] (2013) | 1.00 | 0.00 | 0.56 | 0.89 | 0.33 | 1.00 | 0.81 | 0.66 |
| Kozek-Langenecker et al. [21] (2016) | 0.94 | 0.22 | 0.80 | 1.00 | 0.38 | 0.50 | 0.88 | 0.67 |
| Steffel et al. [22] (2018) | 0.97 | 0.17 | 0.50 | 0.97 | 0.81 | 0.79 | 0.63 | 0.69 |
| Clinical Excellence Commission [3] (2018) | 1.00 | 0.53 | 0.31 | 1.00 | 0.73 | 0.50 | 0.81 | 0.70 |
| Bono et al. [23] (2009) | 1.00 | 0.44 | 0.98 | 1.00 | 0.27 | 0.63 | 0.75 | 0.72 |
| Douketis et al. [5] (2012) | 1.00 | 0.00 | 0.58 | 1.00 | 0.92 | 1.00 | 0.75 | 0.75 |
| Doherty et al. [10] (2017) | 1.00 | 0.47 | 0.65 | 1.00 | 0.60 | 0.83 | 0.75 | 0.76 |
| Kristensen et al. [24] (2014) | 1.00 | 0.75 | 0.55 | 1.00 | 0.65 | 0.67 | 0.75 | 0.77 |
| Narouze et al. [25] (2018) | 0.94 | 0.58 | 0.78 | 0.97 | 0.56 | 0.83 | 0.81 | 0.78 |
| Fleisher et al. [26] (2014) | 1.00 | 0.67 | 0.88 | 1.00 | 0.65 | 0.67 | 0.75 | 0.80 |
| Valgimigli et al. [27] (2018) | 1.00 | 0.78 | 0.86 | 1.00 | 0.92 | 0.92 | 0.75 | 0.89 |
| De Hert et al. [4] (2018) | 1.00 | 0.75 | 0.93 | 1.00 | 0.83 | 1.00 | 0.75 | 0.89 |
Table 3
Summary Appraisal of Guidelines for Research and Evaluation II scores of the gathered clinical practice guidelines
Table 4
Non-parametric analysis of variance and pairwise comparisons between the Appraisal of Guidelines for Research and Evaluation II domains showed that there were significant differences between selected domains
Table 5
Interobserver agreement
| Weighted Cohen’s kappa | |
|---|---|
| No. | 16 |
| Missing | 0 |
| Median | 0.840 |
| Minimum | 0.190 |
| Maximum | 1.00 |
| 25th percentile | 0.665 |
| 50th percentile | 0.840 |
| 75th percentile | 0.982 |
Table 6
Classification of the potential risk of serious bleeding in elective spinal procedures
Table 7
Perioperative management of antithrombotics in elective spinal procedures: general considerations
Table 8
Perioperative management of aspirin and aspirin combinations therapy, P2Y12 and phosphodiesterase inhibitors, non-ASA NSAIDS, and anticoagulant agents in elective spinal procedures
References
1. Tafur A, Douketis J. Perioperative management of anticoagulant and antiplatelet therapy. Heart 2018 104:1461–7.


2. Dimitropoulos K, Omar MI, Chalkias A, Arnaoutoglou E, Douketis J, Gravas S. Perioperative antithrombotic (antiplatelet and anticoagulant) therapy in urological practice: a critical assessment and summary of the clinical practice guidelines. World J Urol 2020 38:2761–70.



3. Clinical Excellence Commission. Guidelines on perioperative management of anticoagulant and antiplatelet agents [Internet] Sydney: Clinical Excellence Commission. 2018 [cited 2022 Jul 5]. Available from: https://www.cec.health.nsw.gov.au/__data/assets/pdf_file/0006/458988/Guidelines-on-perioperative-management-of-anticoagulant-and-antiplatelet-agents.pdf
4. De Hert S, Staender S, Fritsch G, et al. Pre-operative evaluation of adults undergoing elective noncardiac surgery: updated guideline from the European Society of Anaesthesiology. Eur J Anaesthesiol 2018 35:407–65.

5. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012 141(2 Suppl): e326S–e350S.


6. Spencer NH, Sardo LA, Cordell JP, Douketis JD. Structure and function of a perioperative anticoagulation management clinic. Thromb Res 2019 182:167–74.


7. Spyropoulos AC, Brohi K, Caprini J, et al. Scientific and Standardization Committee Communication: guidance document on the periprocedural management of patients on chronic oral anticoagulant therapy: recommendations for standardized reporting of procedural/surgical bleed risk and patient-specific thromboembolic risk. J Thromb Haemost 2019 17:1966–72.



8. Madhugiri VS, Singh V, Kishore K, Reddy A, Bysani P. Current practice in neurosciences: peri-operative management of patients on anti-platelet agents and anti-coagulants and prophylaxis for venous thrombo-embolism in the neurosurgical setting: summary of evidence and practice guidelines [Internet] New Delhi: Neurology India. 2019 [cited 2022 Jul 5]. Available from: https://www.neurologyindia.com/documents/NI_May%20June%20CPIN%202019.pdf
10. Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol 2017 69:871–98.

11. Boet S, Etherington C, Larrigan S, et al. Measuring the teamwork performance of teams in crisis situations: a systematic review of assessment tools and their measurement properties. BMJ Qual Saf 2019 28:327–37.


12. Douketis J, Cervi A. Managing patients who are receiving warfarin or a direct oral anticoagulant and need an elective surgery or procedure. Blood Adv 2019 3:1925.




13. Moia M, Squizzato A. Reversal agents for oral anticoagulant-associated major or life-threatening bleeding. Intern Emerg Med 2019 14:1233–9.




14. Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev Med 2010 51:421–4.


15. AGREE Next Steps Consortium. AGREE Reporting Checklist [Internet] [place unknown]: AGREE Next Steps Consortium. 2016 [cited 2022 Jul 5]. Available from: https://www.agreetrust.org/resource-centre/agree-reporting-checklist/
16. R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2018.
17. Albaladejo P, Bonhomme F, Blais N, et al. Management of direct oral anticoagulants in patients undergoing elective surgeries and invasive procedures: updated guidelines from the French Working Group on Perioperative Hemostasis (GIHP): September 2015. Anaesth Crit Care Pain Med 2017 36:73–6.


18. Godier A, Fontana P, Motte S, et al. Management of antiplatelet therapy in patients undergoing elective invasive procedures: proposals from the French Working Group on Perioperative Haemostasis (GIHP) and the French Study Group on Thrombosis and Haemostasis (GFHT): in collaboration with the French Society for Anaesthesia and Intensive Care Medicine (SFAR). Anaesth Crit Care Pain Med 2018 37:379–89.

19. Rossini R, Tarantini G, Musumeci G, et al. A multidisciplinary approach on the perioperative antithrombotic management of patients with coronary stents undergoing surgery: surgery after stenting 2. JACC Cardiovasc Interv 2018 11:417–34.

20. Armstrong MJ, Gronseth G, Anderson DC, et al. Summary of evidence-based guideline: periprocedural management of antithrombotic medications in patients with ischemic cerebrovascular disease: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013 80:2065–9.



21. Kozek-Langenecker SA, Ahmed AB, Afshari A, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology: first update 2016. Eur J Anaesthesiol 2017 34:332–95.

22. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J 2018 39:1330–93.

23. Bono CM, Watters WC 3rd, Heggeness MH, et al. North American Spine Society evidence-based clinical guidelines for multidisciplinary spine care: antithrombotic therapies in spine surgery [Internet]. Burr Ridge (IL): North American Spine Society. 2009 [cited 2022 Jul 5]. Available from: https://www.spine.org/Portals/0/Assets/Downloads/ResearchClinicalCare/Guidelines/AntithromboticTherapies.pdf
24. Kristensen SD, Knuuti J, Saraste A, et al. 2014 ESC/ESA guidelines on non-cardiac surgery: cardiovascular assessment and management: the Joint Task Force on non-cardiac surgery: cardiovascular assessment and management of the European Society of Cardiology (ESC) and the European Society of Anaesthesiology (ESA). Eur J Anaesthesiol 2014 31:517–73.

25. Narouze S, Benzon HT, Provenzano D, et al. Interventional spine and pain procedures in patients on antiplatelet and anticoagulant medications (second edition): guidelines from the American Society of Regional Anesthesia and Pain Medicine, the European Society of Regional Anaesthesia and Pain Therapy, the American Academy of Pain Medicine, the International Neuromodulation Society, the North American Neuromodulation Society, and the World Institute of Pain. Reg Anesth Pain Med 2018 43:225–62.

26. Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014 130:2215–45.


27. Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: the Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2018 39:213–60.

- TOOLS