Selective Estrogen Receptor Modulators
Article information
Abstract
Selective estrogen receptor modulators (SERMs) are now being used as a treatment for breast cancer, osteoporosis and postmenopausal symptoms, as these drugs have features that can act as an estrogen agonist and an antagonist, depending on the target tissue. After tamoxifen, raloxifene, lasofoxifene and bazedoxifene SERMs have been developed and used for treatment. The clinically decisive difference among these drugs (i.e., the key difference) is their endometrial safety. Compared to bisphosphonate drug formulations for osteoporosis, SERMs are to be used primarily in postmenopausal women of younger age and are particularly recommended if there is a family history of invasive breast cancer, as their use greatly reduces the incidence of this type of cancer in women. Among the above mentioned SERMs, raloxifene has been widely used in prevention and treatment of postmenopausal osteoporosis and vertebral compression fractures, and clinical studies are now underway to test the comparative advantages of raloxifene with those of bazedoxifene, a more recently developed SERM. Research on a number of adverse side effects of SERM agents is being performed to determine the long-term safety of this class of compouds for treatment of osteoporosis.
Introduction
Due to the reduction in estrogen levels after menopause, hot flashes and profuse sweating occur as part of the menopausal vasomotor symptoms; this is along with estrogen-related urogenital atrophy and loss of bone density that may lead to osteoporosis. Postmenopausal hormone therapy is used to address some of these symptoms, but due to the heightened risk of breast cancer, endometrial cancer, thromboembolism and vaginal bleeding from estrogen therapy, the use of hormone therapy may be limited [1]. For postmenopausal women, selective estrogen receptor modulators (SERMs) drugs act as partial estrogen receptors agonists for maintaining bone density bone for applications in osteoporosis treatment and at the same time act as estrogen receptor antagonists in breast tissues for applications in breast cancer prevention in women, along with effects on the uterus and vagina that depend on their interaction with the estrogen receptors in target tissues.
Tamoxifen, a first generation breast cancer drug treatment, was developed in the 1970s, and it is currently prescribed for estrogen receptor positive (ER+) breast cancer. On breast tissue, tamoxifen acts as an estrogen receptor antagonist, but in bone tissue, it acts as an agonist, being able to maintain bone mineral density (BMD) in postmenopausal women. As such, tamoxifen was also considered for treatment for osteoporosis; however, tamoxifen can act as an agonist for uterus endometrial hyperplasia and polyp production, thus possibly increasing the risk for endometrial cancer, and negating its use for osteoporosis [2]. There has been a second-generation osteoporosis drug, raloxifene, also an estrogen receptor antagonist and, unlike tamoxifen, it is anti-estrogen like on the on the uterus and was approved by the Food and Drug Administration to treat osteoporosis. The third-generation drug, bazedoxifene, has also been developed to treat osteoporosis and has similar effects to that of raloxifene [3].
Much research concerning using SERMs to treat osteoporosis has been published, especially comparative studies for raloxifene, bazedoxifene and bisphosphonates (BP). The cardiovascular effects and effects on the lipid profile must also be carefully studied to determine long-term safety.
Mechanism of Action
The mechanism of action of SERM class of compounds relies on their tissue-selective estrogen receptor agonist or antagonist activity in their interaction with the estrogen receptor, and these properties encompass a certain level of molecular and functional complexity. The estrogen receptor has two subunits (α and β chains), and SERMs interact with either of these subunits, and from this interaction, there is a certain level of target-site specificity and tissue-specificity for SERM action [4]. This differential behavior of SERMs depends on eliciting varying signaling properties from the estrogen receptor that is tissue specific, and such effects have profound physiological effects and are not dictated at the DNA level [5].
With regards to bone loss and osteoporosis, the action of SERMs on the estrogen receptor affects bone homeostasis by downmodulating the activity of osteoclasts in a transforming growth factor-β3-dependent manner and reducing bone resorption. This affect allows in preventing and treating osteoporosis.
Review of Clinical Data
1. Efficacy of SERMs in clinical trials
The multiple outcomes for raloxifene evaluation (MORE) study was a multicenter, randomized, blinded, placebo-controlled clinical trial, conducted for over three years for participants on raloxifene. The trial was able to demonstrate the effectiveness of raloxifene in maintaining BMD and minimizing bone loss in both the spine and femur neck for the study participants. Postmenopausal women with osteoporosis achieved a reduction of 30% in vertebral fractures with raloxifene. However, for non-vertebral fractures, there was no reduction with raloxifene use [6]. For lasofoxifene, another SERM, the randomized, double-blind, placebo-controlled study Postmenopausal Evaluation and Risk Reduction with Lasofoxifene (PEARL) clinical trial for vertebral fracture risk in postmenopausal women showed a 42% reduction [7]. In another study, for the bazedoxifene 20 mg group and the raloxifene 60 mg group compared with the placebo group, there was a significant effect on maintaining BMD and reducing annual new vertebral fractures [8].
Many clinical trials with postmenopausal osteoporotic women examining various SERM preparations have shown that SERMs can maintain BMD and reduced incidence of vertebral fractures, but did not reduced fracture risk in non-vertebral cases, indicating that their benefit for fractures is anatomically limited.
2. Extra-skeletal effects
The potential cardiovascular effects for raloxifene were studied by looking at the blood lipid profile changes for participants taking the medication, and a reduction in the high dose lipoprotein cholesterol was seen, but without a triglyceride increasing effect. The MORE study found an increased risk of a cardiovascular event in women [9]; however, the Raloxifene Use for the Heart (RUTH) indicated cardiovascular disease risk changes compared to placebo that were not statistically significant [9].
For breast cancer risk for participants taking raloxifene, the MORE study found that over a period of four years, there was 62% reduction in incidence of breast cancer. In the RUTH study, there was also a reduction of risk of invasive breast cancer by 44% relative risk reduction when used for an average of 5.6 years [9]. The continuing outcomes relevant to evista study, which took place over an eight-year period, found a 66% relative risk reduction [10].
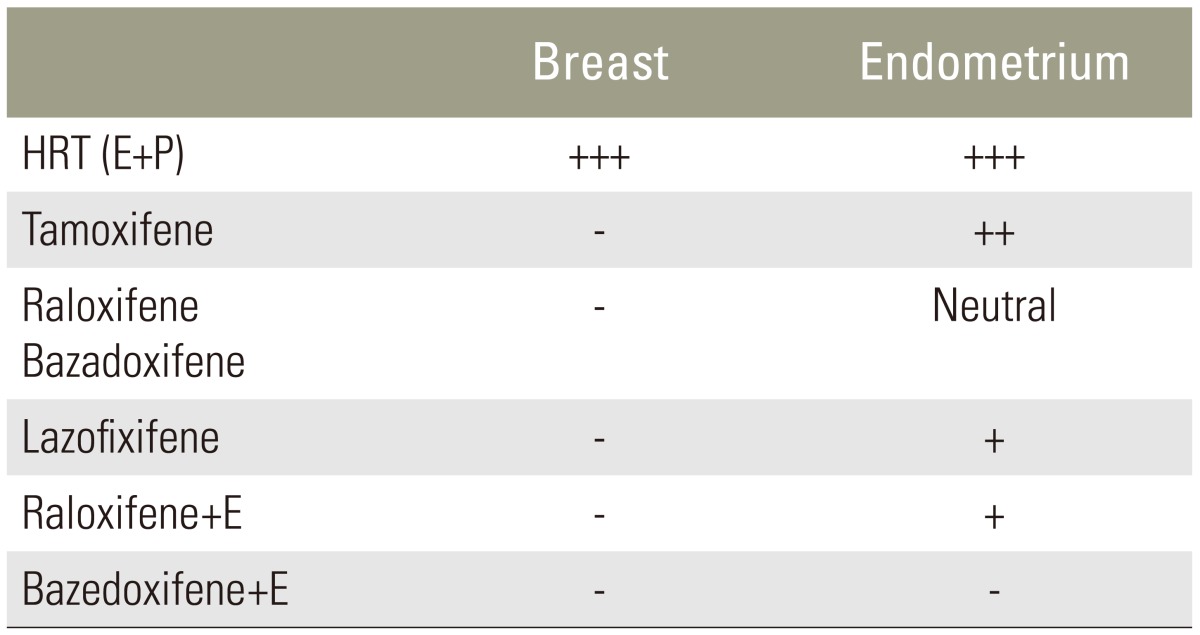
In the case of tamoxifen and the endometrial effects on the vaginal mucosa, the endometrial cancer risk was found to be 2.5 times higher if raloxifene was used. Bazedoxifene has been found to have no net effect on endometrial cancer occurrence and does not increase its risk. For lasofoxifene, it has a slightly positive effect on the increase of endometrial thickness, but has a low endometrial cancer incidence [3]. Thus, the key difference between these SERMs is their endometrial safety. A tissue selective estrogen complex (TSEC) was developed in accordance with the endometrial effect, and it is combined with the presence or absence of the estrogen conjugate (Table 1).
3. Raloxifene vs. bazedoxifene
According to a bazedoxifene 20 mg, bazedoxifene 40 mg, raloxifene 60 mg per day after 36 months and placebo-controlled study, vertebral fracture risk was significantly lower in the study population of postmenopausal women with osteoporosis, as compared to placebo. For the bazedoxifene versus raloxifene arms, there were no statistically significant differences in efficacy. However, for the high risk group with the femoral neck T-score < or= -3.0, and the group with T-score >or= 1 with one or more moderate or severe vertebral fractures, the bazedoxifene 20 mg group had a significant reduction in risk of non-vertebral fractures as well compared to the other arms [3]. A randomized, double blind, placebo-active controlled study to compare the cost-effectiveness of bazedoxifene and raloxifene, bazedoxifene was more cost effective [11]. In addition to breast studies, there appears to be similar effects for raloxifene and bazedoxifene in terms of endometrial safety and blood lipid profiles.
4. Patient compliance
Many osteoporosis drugs have reported improvements in BMD and prevention of fractures in osteoporosis cases, but it is difficult to maintain patient compliance for dosing on schedule. Due to the chronic and progressive nature of osteoporosis, the patient cannot see the direct benefits of taking these medications. SERM drug are usually taken with BP and compliance is low due to gastrointestinal problems and other side effects [12]. However, by using BP in a weekly or monthly basis, better compliance has been reported. According to Licata [13], at seven months of being on these medications, the hormone replacement treatment discontinuation rate was at its highest at 26% for medications with BP formulations, while the SERM discontinuation rate was lower at 19%. From the survey by Huas et al. [14], monthly BP had the best compliance, followed by BP taken weekly and then taken on a daily basis. SERM compliance was also similar. Sambrook et al. [15] showed that weekly and monthly usage, rather than daily usage, had better compliance for BP and BP SERM combinations.
5. Use in the elderly population
As vertebral fractures frequently occur in relatively young patients, as compared to hip fractures, SERMs, which have marginal benefits in decreasing frequency of non-vertebral fractures, have been recommended as the first-line of defense for patients between the ages of 60 and 65. As serious complications from long-term BP use, such as occurrence of femoral atypical fractures and osteonecrosis of the jaw, have recently been reported, using SERMs instead is thought to be more appropriate for patients at approximately 60 years of age. It should be noted that most previous clinical trials on SERM for osteoporosis studies have used cohorts of postmenopausal women at approximately 65 years of age or older [16].
According to Cummings et al. [17], who examined the efficacy of administrating raloxifene to patients with an average age of 85.3 years (range, 75–99 years), a significant change of biochemical markers was also observed in cases of such elderly patients. Jacobsen et al. [18] also reported from a randomized, double-blinded and placebo-controlled study that significant BMD improvement and decreases in vertebral fracture rates were observed among female patients over age 70, compared to the placebo group.
6. Adverse effects and long-term safety
Clinical usage of SERM medications can have several side effects. Representative side effects of raloxifene include hot flashes and leg cramps. As such Davies et al. [19] reported that the occurrence frequency of hot flashes was significantly higher among postmenopausal healthy women under 60 who used raloxifene, as compared to the control group. Also, the study reported that no increase in hot flash frequency was observed at six months after beginning of the treatment. Davies et al. [19] argued that leg cramp occurrence was only a mild symptom of raloxifene use and that it was not significant enough to suspend its use. Raloxifene use has also been linked to rare, but serious side effects, such as venous thromboembolism, including deep vein thrombosis (DVT), pulmonary embolism (PE) and retinal vein thrombosis [17].
Higher frequency and extent of menopausal symptoms, including hot flashes and atrophic vaginitis, are the most commonly observed side effects from tamoxifen use. Increased occurrence of vaginal discharge and irregular menses has also been reported among women who use tamoxifen. Retinopathy has also been observed among women who use high-dose tamoxifen, but a less extensive retinal change can occur from time to time in cases of normal dose usage. However, an impact on vision is known to be very rare [20]. Use of tamoxifen has also been linked to a higher occurrence of cataracts. According to Fisher et al., women on tamoxifen experienced cataracts surgery 57% more often than for the control group [21]. Similar to raloxifene, tamoxifen has been reported to increase the risk of thromboembolic events, including DVT and PE [22].
Unlike BP, SERMs do not continue to impact bones and the effects on BMD cannot be expected to continue once the medication is suspended. Hence, long-term use of SERM medication is required for the prevention of osteoporosis. Messalli and Scaffa [23] reported a good overall risk-benefit profile for raloxifene, although it increases the risk of venous thromboembolism and fatal strokes. They argued that a guarded and careful use of the medication is required, accounting for an individual's specific risk factors.
From a sampling of postmenopausal women, Siris et al. [24] compared the skeletal effects of raloxifene with placebo and reported that a decrease in non-vertebral fracture risk was unlikely to persist over eight years. They recommended switching to other anti-osteoporosis agents after an eight-year use of raloxifene.
Conclusions
With the most recent versions of SERMs, they have become suitable for treatment of osteoporosis in postmenopausal women. Although SERMs are effective for preventing vertebral fractures and maintaining BMD, they have certain limitations in preventing non-vertebral fractures. SERMs also have extra-skeletal effects as well as related side effects, and thus require caution in their use. The effects for those over age 70 have also been proven to a certain degree. In terms of patient compliance, SERM use was better adhere to than for BP. Overall, the long-term safety of SERM are acceptable; however, after eight years of usage, side effects are more likely to be observed. Based on these characteristics, SERMs are currently expected to be a good choice for treating osteoporosis in postmenopausal women.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.