Radiologic and Clinical Courses of Degenerative Lumbar Scoliosis (10°–25°) after a Short-Segment Fusion
Article information
Abstract
Study Design
Retrospective study.
Purpose
We report the surgical outcomes of small degenerative lumbar scoliosis (DLS) patients treated by a short-segment fusion and followed for a minimum of 5 years.
Overview of Literature
Several surgical options are available for the treatment of DLS, such as decompression only, decompression plus a short-segment fusion, or decompression with a long segment fusion. Few studies have evaluated the results of a short-segment fusion in patients with DLS over time.
Methods
Seventy small DLS patients (Cobb's angle, 10°–25°) with a minimum follow-up of 5 years were treated with a short-segment fusion between March 2004 and February 2010. The mean patient age was 71 (male:female=16:54), with a follow-up of 6.5 years (range, 5.0–11.6). The Cobb's angle, 1 and 2 segment coronal upper intervertebral angle, 1 and 2 segment sagittal upper intervertebral angle, the lumbar lordosis angle, and the C7 plumb lines (coronal and sagittal) were evaluated using simple radiographs, and visual analog scale (VAS), back pain was assessed preoperatively, immediately after surgery, and at 3, 6, and 12 months and 3 and 5 years after surgery. To identify factors influencing the radiologic progression, age, number of fusion segments, vertebral levels of fusion, body mass index, lowest instrumented vertebra (L5 or S1), bone mineral density (>–2.5, ≤–2.5), and the presence of an interbody fusion were analyzed.
Results
The Cobb's angle and 1 segment coronal upper intervertebral angle showed more progression during follow up, particularly at 6 and 12 months after surgery. Clinical outcomes and radiological results were found to be significantly associated (p=0.041). No statistically significant association was found between other factors affecting radiologic progression from postoperative 6 months to 1 year.
Conclusions
Radiologic variables (the Cobb's angle and coronal upper intervertebral angle–1) should be carefully considered and clinical caution exercised from 6 to 12 months after short-segment fusion in small DLS (10°–25°).
Introduction
The primary symptoms of patients with degenerative lumbar scoliosis (DLS) are severe low back pain, radiating leg pain, and sagittal imbalance. Low back pain may be caused by degenerative arthrosis of facet joints, degenerative changes in the intervertebral discs, or coronal or sagittal imbalance. Radiating leg pain can occur due to spinal stenosis or rotatory subluxation associated with nerve root compression by pedicles. Intermittent claudication is the most common clinical indication following surgical treatment [1]. The goals of the operation are pain alleviation and deformity correction. Surgical options for DLS include decompression alone, decompression combined with a short-segment fusion, and decompression with a long-segment fusion [23]. Decompression only is not usually recommended, as it may worsen instability at the tip of the degenerative curve, increase spinal instability, and exacerbate low back pain. Therefore, fusion and decompression are necessary in most cases. Long fusion is considered more effective for deformity correction when the spinal curve is >40° or spinal stenosis is associated. As compared to long-segment fixation, the advantages of short-segment fusion are a shorter operation time with fewer postoperative complications. However, degenerative scoliosis is more common in older adults with associated medical conditions [4]. Even though a short fusion shortened operation times, reduced blood loss, and reduced the incidence of postoperative complications, few long-term studies have been conducted on its effectiveness. The purpose of this study was to explore the impact of a shortsegment fusion on the clinical and radiological outcomes with a minimum of 5-year follow-up in DLS patients treated by a short-segment fusion.
Materials and Methods
We retrospectively reviewed 70 patients who underwent decompression and a short-segment fusion, due to degenerative scoliosis. These patients had Cobb's angles ranging between 10° and 25° and required surgical treatment, due to persistent low back pain and radiating leg pain, despite a minimum of 6 months of conservative treatment between March 2004 and February 2010. All operations were performed by a single orthopedic spine specialist. Those with a systemic condition (including medical conditions), those at operative risk due to underlying diseases, those requiring a long fusion for the correction of deformities (Cobb's angles >25°), and those with severe coronal and sagittal imbalance or spinal stenosis were excluded. The subjects were 12 men and 58 women with an overall mean age of 71.00±7.57 years (range, 61–82 years). The average follow-up period was 78±19 months (range, 60–134 months). The short fusion was performed at one or two levels in patients with degenerative scoliosis of 10°–25°. The range of fusion was defined as scoliosis involving deformity confined to the upper spine. Decompression, including laminectomy, was conducted on all patients, and posterolateral spinal fusion using screws was conducted. A Mega Spine device (BK Meditech, Seoul, Korea) was used in all patients. Interbody fusion was selectively performed on 18 patients with high segmental instability and narrow spaces between the intervertebral bodies. This procedure was not carried out in patients with moderately maintained intervertebral disc heights and low segmental instability. Radiological and clinical results were assessed preoperatively, immediately after surgery, and at 3, 6, and 12 months and 3 and 5 years after surgery. We sought to identify the degrees of correction loss during follow-up and to examine changes in the symptoms. Clinical assessments were performed using the visual analog scales (VAS) for low back pain at our outpatient clinic and results were obtained from the medical records. For radiological assessments, anteroposterior and lateral X-ray images of the lumbar and entire spine were taken using a 14×36 in cassette in a standing position, and the Cobb's angle, coronal upper intervertebral angle (UIA), and sagittal upper intervertebral angles were measured as differences between the angles formed by upper apical vertebrae and the endplates of the fused segments (UIA-1 and -2) (Fig. 1). Lumbar lordosis angles were measured between the upper endplate of T12 and that of S1. Coronal and sagittal imbalance was determined by a plumb line drawn from the center of C7 through the posterosuperior aspect of S1. To identify factors influencing radiologic progression, age, number of fusion segments, vertebral levels of fusion, body mass index (BMI), lowest instrumented vertebra (L5 or S1), bone mineral density (BMD, >–2.5; ≤–2.5), and the presence of interbody fusion were analyzed. All radiological measurements are the mean values of measures obtained by two orthopedic spine specialists. Measures were compared to identify changes in the degree of correction loss and pain.

Preoperative (A), postoperative (B) and 5-year follow-up (C) standing anteroposterior radiographs of the lumbar spine in a patient treated with short segment fusion. UIA, upper intervertebral angle.
The statistical analysis was performed using SPSS ver. 22.0 (IBM Corp., Armonk, NY, USA). A paired t-test and the repeated measures ANOVA were used to determine the significances of differences. Statistical significance was accepted for p-values of <0.05.
Results
1. Radiologic evaluation (Table 1)
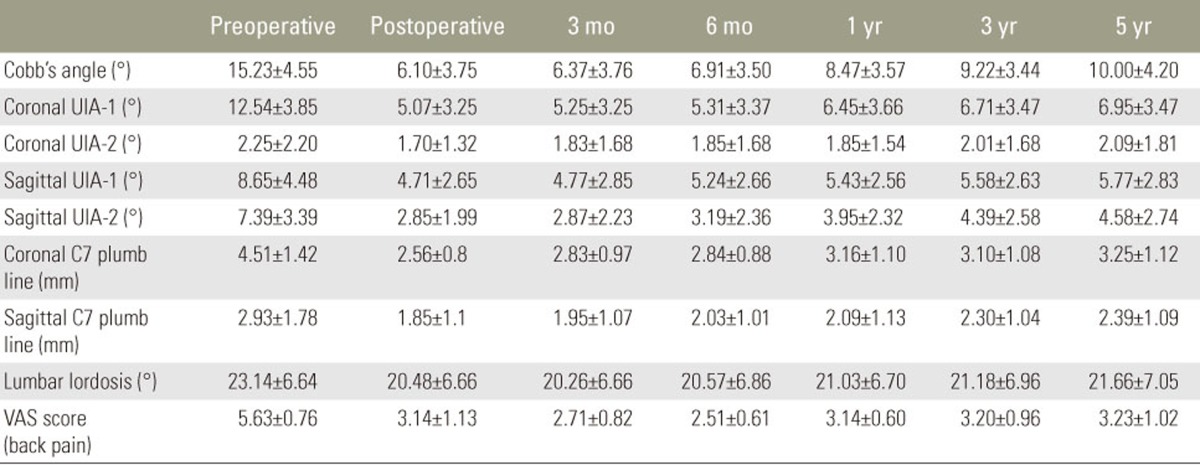
Radiologic parameters of short segment fusion on degenerative lumbar scoliosis for long-term follow-up
1) Cobb's angle
The mean preoperative, immediate post-\operative, and latest follow-up Cobb's angles were 15.23°±4.55°, 6.1°±3.75°, and 10.0°±4.2°, respectively. The mean change in Cobb's angle was –0.27°±0.41° between immediately and 3 months after surgery, –0.54°±0.74° between 3 and 6 months postoperative, –1.56°±3.06° between 6 and 12 months postoperative, 0.75°±1.13° between 12 months and 3 years postoperative, and –0.78°±2.22° between 3 and -5 years postoperative.
2) Coronal UIA (–1 and –2)
The mean preoperative, immediate postoperative, and final follow-up in the 1 segmental coronal UIA-1 were 12.54°±3.85°, 5.07°±3.25°, and 6.95°±3.47°, respectively. The mean changes in the 1 segmental coronal UIA-1 were –0.18°±0.69° between immediately and 3 months after surgery, –0.06°±0.77° between 3 and 6 months postoperative, –1.13°±2.46° between 6 and 12 months postoperative, –0.26°±0.52° from 1 year to 3 years postoperative, and –0.24°±0.64° between 3 and 5 years postoperative. The mean preoperative, immediate postoperative, and last follow-up 2 segmental coronal upper intervertebral angle (UIA-2) were 2.25°±2.20°, 1.70°±1.32°, and 2.09°±1.81°, respectively. Mean changes in the 2 segmental coronal UIA-2 were –0.13°±0.47° between immediately and 3 months after surgery, –0.02°±0.11° between 3 and 6 months postoperative, 0°±0.26° between 6 and 12 months postoperative, –0.16°±0.43° from 1 year to 3 years postoperative, and –0.08°±0.38° between 3 and 5 years postoperative.
3) Sagittal upper intervertebral angle (UIA-1 and -2)
The mean preoperative, immediate postoperative, and latest follow-up 1 sagittal UIA-1 were 8.65°±4.48°, 4.71°±2.65°, and 5.77°±2.83°, respectively. The mean changes in 1 sagittal UIA-1 were -0.06°±0.77° between immediately and 3 months after surgery, –0.47°±0.53° between 3 and 6 months postoperative, –0.19°±0.76° between 6 months and 1 year postoperative, –0.15°±0.26° from 12 months to 3 years postoperative, and –0.19°±0.58° between 3 and 5 years postoperative. The mean preoperative, immediate postoperative, and latest follow-up 2 sagittal UIA-2 were 7.39°±3.39°, 2.85°±1.99°, and 4.58°±2.74°, respectively. The mean changes in 2 sagittal UIA-2 were –0.02°±0.62° between immediately and 3 months after surgery, –0.33°±0.71° between 3 and 6 months postoperative, –0.76°±1.23° between 6 months and 1 year postoperative, –0.43°±1.03° from 12 months to 3 years postoperative, and –0.19°±0.53° between 3 and 5 years postoperative.
4) Lumbar lordosis
The mean preoperative, immediate postoperative, and last follow-up on lumbar lordosis angles were 23.14°±1.78°, 20.48°±6.66°, and 21.66°±7.05°, respectively. The mean changes during follow-up were 0.23°±1.11° between immediately and 3 months after surgery, –0.31°±0.63° between 3 and 6 months postoperative, –0.46°±0.89° between 6 and 12 months postoperative, –0.15°±0.76° between 12 months and 3 years postoperative, and –0.49°±1.02° between 3 and 5 years postoperative.
5) Coronal C7 plumb line
The mean coronal C7 plumb line (cm) was 4.51±1.42 cm preoperatively, 2.56±0.8 cm immediately after surgery, and 3.25±1.12 cm at the last follow-up. The mean changes during follow-up were –0.27±0.34 cm between immediately and 3 months after surgery, –0.01±0.38 cm between 3 and 6 months postoperative, –0.32±0.65 cm between 6 and 12 months postoperative, 0.06±0.35 cm between 12 months 3 years postoperative, and –0.15±0.25 cm between 3 and 5 years postoperative.
6) Sagittal C7 plumb line
The mean sagittal C7 plumb line (cm) was 2.93±1.78 cm preoperatively, 1.85±1.1 cm immediately after surgery, and 2.39±1.09 cm at the last follow-up. The mean changes during follow-up were –0.27±0.34 cm between immediately and 3 months after surgery, –0.01±0.38 cm between 3 and 6 months postoperative, –0.32±0.65 cm between 6 and 12 months postoperative, 0.06±0.35 cm between 12 months and 3 years postoperative, and –0.15±0.25 cm between 3 and 5 years postoperative.
The changes in the radiological parameters indicated postoperative improvements in angles and radiologic progression over time. In particular, the change in radiologic progression between 6 months and 1 year was the largest, and the increases in Cobb's and coronal UIA-1 varied widely (Table 1).
2. Clinical evaluation (VAS)
The mean VAS scores were 5.63±0.76 preoperatively, 3.14±1.13 immediately after surgery, and 3.22±1.02 at the last follow-up. The mean changes in the VAS scores were 0.43±1.08 between immediately and 3 months after surgery, 0.20±0.71 between 3 and 6 months postoperative, –0.63±0.64 between 6 and 12 months postoperative, –0.06±0.72 from 12 months to 3 years postoperative, and –0.03±0.51 between 3 and 5 years postoperative.
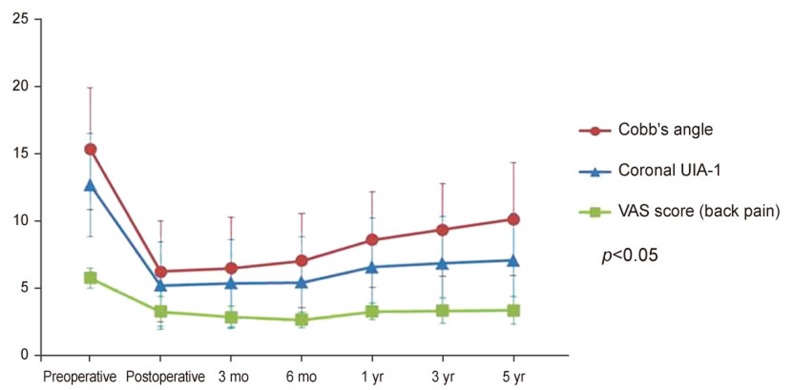
VAS scores showed a decrease immediately after surgery and then gradually increased. As observed for Cobb's and the coronal 1 segment UIA-1, the VAS scores showed the largest increases between 6 and 12 months postoperative. Repeated measures ANOVA indicated that radiologic changes and clinical outcomes were significantly correlated (p=0.041) (Fig. 2).
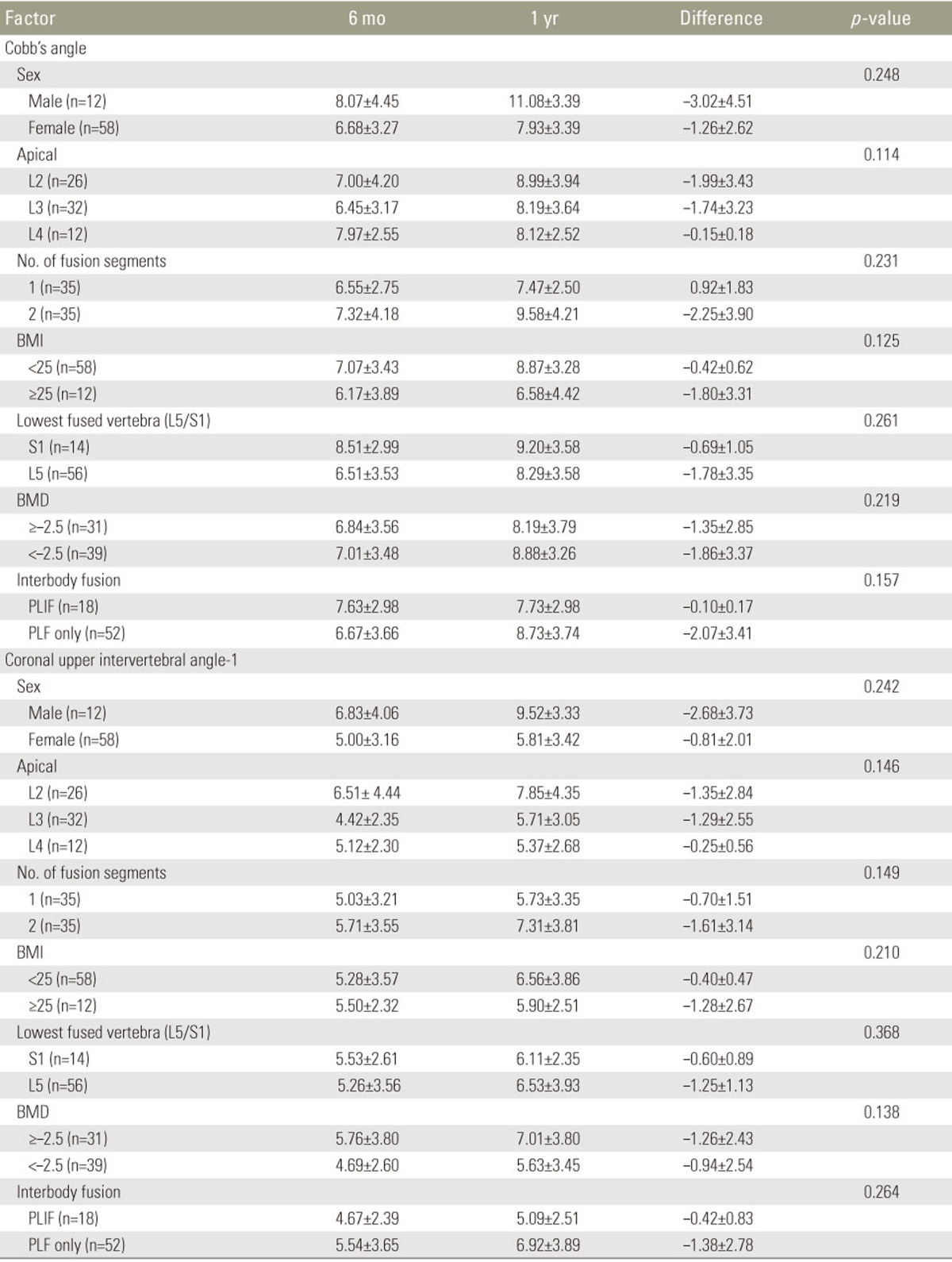
3. Factors affecting radiologic progression (Cobb's angle, coronal UIA-1)
The factors affecting Cobb's angle and coronal UIA-1 of the study subjects are shown in Table 2. For male patients, the mean changes in correction loss between 6 and 12 months post-op. were –3.02°±4.51° for Cobb's angle and 2.68°±3.73° for the coronal UIA-1. For female patients, the changes were –1.26°±2.62° for Cobb's angle and 0.81°±2.01° for the coronal UIA-1.
For the vertebral levels of fusion, there were 26 L2 level cases, 32 L3 level cases, and 12 L4 level cases. The mean changes in correction loss between 6 and 12 months postoperative were as follows: Cobb's angle of –1.99°±3.43° and the coronal UIA-1 of –1.35°±2.84° at L2, Cobb's angle of 1.74°±3.23° and the coronal UIA-1 of –1.29°±2.55° at L3, and a Cobb's angle of 0.15°±0.18° and the coronal UIA-1 of –0.25°±0.56° at the L4 level.
Short fusion was performed at a single level or at two levels in 35 cases. The mean change in correction loss between 6 and 12 months was Cobb's angle of –0.92°±1.83° and the coronal UIA-1 of –0.70°±1.51° for a single-level fusion and a Cobb's angle of –2.25°±3.90° and a coronal UIA-1 of –1.61°±3.14° for a two-level fusion.
Obesity was defined as a BMI >25 kg/m2 and normal weight as a BMI of <25 kg/m2. Twelve patients had a BMI of >25 and 58 had a BMI of <25. For obese patients, the mean changes in the correction loss between 6 and 12 months were a Cobb's angle of –0.42°±0.62° and a coronal UIA-1 of –0.40°±0.47°, while for normal-weight patients, a Cobb's angle of –1.80°±3.31° and coronal UIA-1 of –1.28°±2.67°.
Patients were also dichotomized based upon the lowest instrumented vertebra position (L5 or S1). There were 14 cases of S1 and 56 cases of L5. For S1, the changes in correction loss between 6 and 12 months were a Cobb's angle of –0.69°±1.05° and a coronal UIA-1 of –0.59°±0.87°. For L5, changes were a Cobb's angle of –1.78°±3.35° and a coronal UIA-1 of –1.27°±2.71°.
Patients were also divided into osteoporotic and non-osteoporotic groups based on the BMD densities (T-score ≤–2.5 or >–2.5). There were 31 osteoporotic patients and 39 non-osteoporotic patients. For the non-osteoporotic patients, the mean changes in correction loss between 6 and 12 months postoperative were a Cobb's angle of –1.35°±2.85° and a coronal UIA-1 of –1.26°±2.43°, while for osteoporotic patients, a Cobb's angle of –1.86°±3.37° and a coronal UIA-1 of –0.94°±2.54°.
The 70 patients were also divided into performance (n=18) and non-performance (n=52) groups based on the achievement of interbody fusion. In the performance group, the mean changes in correction loss between 6 and 12 months postoperative were a Cobb's angle of –0.10°±0.17° and a coronal UIA-1 of –0.42°±0.83°, while for the non-performance group, mean changes in the Cobb's angle of –2.07°±3.41° and a coronal UIA-1 of –1.38°±2.78°.
4. Complications
Complications occurred in 5 of the 70 patients. One patient with a compression fracture of the upper lumbar spine, due to adjacent segment disease, was managed conservatively. Four patients required removal of the screws. Two of them were treated by a long fusion during the revision surgery based upon severe symptoms.
Discussion
The three major symptoms of patients with DLS are low back pain, radiating leg pain, and sagittal imbalance. Unlike spinal stenosis, low back pain persists even at rest while sitting, due to severe degenerative changes or DLS progression [5]. In addition to back pain, radiating pain in the lower limbs is severe and primarily results from nerve compression caused by spinal stenosis and a lateral transposition of the vertebral body. Furthermore, sagittal imbalance or rotatory subluxation can generate back pain. Spinal deformity is often associated with coronal imbalance caused by scoliosis and sagittal imbalance. Surgical interventions are dependent upon the symptoms. Treatment modalities for DLS include decompression alone, decompression combined with short-segment fusion to partially correct the deformity, and decompression coupled with long-segment fusion to correct the entire deformity. Epstein et al. [6] noted that effective management and functional recovery of radiating pain were achieved by surgical decompression of the lateral recess alone. Moon et al. [7] also performed decompression alone when nerve compression involved only one spinal segment with favorable clinical outcomes. Decompression surgery alone was not recommended since instability may worsen in the proximal adjacent segment of the degenerative curve [89]. Vaccaro and Ball [10] suggested that decompression alone can lead to instability and aggravate low back pain in the future. Decompression combined with a short-segment fusion is usually applied to patients with a small Cobb's angle or a mild lateral transposition of the vertebral body. Tribus [3] recommended fusion of decompressed segments in patients without coronal or sagittal imbalance, and proposed long-segment fusion for patients with a large Cobb's angle or coronal or sagittal imbalance. Cho et al. [11] reported that long fusion and instrumentation are more effective than short fusion for correcting scoliotic angle and coronal imbalances, and considered long fusion more effective than short fusion in patients with a Cobb's angle of >25°. In contrast, Simmons [9] stated that long fusion was unnecessary to correct scoliosis deformities and that back pain and stenosis may disappear when the spinal balance is maintained by a short fusion. Hwang et al. [12] investigated radiologic and degenerative changes in patients with a minimum follow-up of 3 years, and found that decompression combined with a short fusion improved symptoms and did not accelerate degenerative changes. In the present study, we explored mid- and long-term postoperative results by examining radiologic and clinical changes after a minimum follow-up of 5 years in patients who underwent a short fusion. Based on previous studies, patients were divided into a short-segment fusion and a long-segment fusion based on a Cobb's angle of 25°. Small DLS was defined as a DLS with a Cobb's angle between 10° and 25° [11]. We aimed to identify changes immediately and subsequently after surgery by measuring the Cobb's angle, 1 and 2 segment coronal UIA-1 and -2, 1 and 2 segment sagittal UIA-1 and -2, lumbar lordotic curves, and coronal and sagittal vertical axes (the coronal and sagittal C7 plumb line) based upon radiological outcomes. Cobb's angles were changed due to apical disc degeneration, and this could have degenerative effects even at UIA-2.
In most cases, a substantial improvement was observed for Cobb's angle correction in the coronal plane, coronal UIA-1 and -2, coronal vertical axis, lumbar lordotic angle in the sagittal plane, the sagittal vertical axis, and the sagittal UIA-1 and -2 as compared to preoperative angles. VAS pain scores improved, in most patients, immediately after surgery. According to Jackson and Simmons [13], pain was reduced by 93% in patients with degenerative scoliosis associated with intervertebral disc stenosis after decompression and a pedicle screw fixation. These authors performed decompression and laminectomy in all patients, as well as spinal fusion surgery with screws. Based on their long-term follow-up results, progressive radiologic correction loss and pain intensification occurred over time postoperatively. Iizuka et al. [14] noted that the Cobb angle was significantly correlated with EuroQol-5D utility scores and EuroQol-VAS scores. In particular, radiologic progression, as determined by the Cobb's angle and the coronal UIA-1, was highest between 6 and 12 months after surgery. VAS scores increased similarly, indicating an association between these two angles and clinical symptoms (p<0.05) (Fig. 1). In the present study, the largest increase in the correction loss occurred between 6 and 12 months after surgery, which concurs with the findings of Cho et al. [11] and Hwang et al. [12].
According to previous studies, DLS is more common in women (range, 52%–71%) [5151617]. In the present study, the proportion of female patients was greater at 83%, and during the 5-year follow-up, the Cobb's and coronal UIA-1 progressively increased in men and women between 6 and 12 months after surgery. Furthermore, the rate of progression was higher in men. In order to identify the association between the BMI and radiologic parameter progression, we divided patients into two groups about a cut-off BMI of 25 kg/m2. Radiologic progression was found to be greatest between 6 and 12 months post-op in both groups. In contrast, a previous study failed to reveal any association between BMI and radiologic parameter progression [1], and the present study also found no association between BMI and progression. Since individual radiologic changes may differ considerably after surgery due to osteoporotic changes in DLS patients, preoperative BMDs were measured in all patients. Using a T-score of –2.5 as a standard value, we compared the radiologic progressions during specific time periods. Progression was found to be the greatest between 6 and 12 months after surgery, but no significant differences were found between the “osteoporotic” and “non-osteoporotic” groups.
In the present study, short fusion was defined as the fusion of 1 or 2 segments, and the effect of number of fused segments on the progression of radiologic parameters was examined. With respect to changes in the Cobb's and coronal UIA-1 between 6 and 12 months after surgery, rates of loss were higher for two-segment fusions (–2.25°±3.9° vs. –0.92°±1.83°) compared to one-segment fusions. To assess the postoperative progression of radiologic parameters based on vertebral fusion levels, we examined the rates of progression in patients who underwent fusion at the L2, L3, or L4 vertebral level. Radiologic parameter progression was higher between 6 and 12 months after surgery at L2 and L3, but higher between 3 and 5 years after surgery at L4. Moon et al. [7] reported that the rate of DLS associated with spinal stenosis was 62% at L3–4 and 92% at L4–5. Moreover, Kim et al. [18] reported that the incidence of spondylolisthesis or retrolisthesis was 34% at L3–4, 56% at L4–5, and 35% at L5–S1. They concluded that degenerative scoliosis at L4–5 is the most important pathogenic factor. In patients with DLS, spinal stenosis and vertebral body displacement most commonly occurs at L4–5, and degenerative facet disease is most frequently found at L4–5.
Bridwell et al. [19] suggested that long fusion requires extension of the device fixation L3 through L5 and extension of the fusion to S1 when sagittal imbalance is associated, due to the degenerative changes commonly associated with the L3–4 and L4–5 levels. However, fusion to the sacrum has the disadvantages of more complicated surgical procedures and an increased risk of non-union at the lumbar/sacral boundary. Cho et al. [11] reported that secondary degenerative changes were commonly observed between L5 and S1 during follow-up. Aebi [8] reported that the non-union did not occur after additional posterior interbody fusion at the lumbar/sacral junction. In the present study, 14 patients underwent fusion up to S1 as the lowest instrumented vertebra position, and their radiologic progressions were less severe than those of the 56 patients who underwent fusion to L5. These results suggest that short fusions up to the S1 levels are effective in cases of spinal stenosis or deformity.
Higher complication rates have been reported after surgical treatment for DLS. Studies have reported a wide range of non-union incidences (7%–41%) [1920]. One of the disadvantages of a short fusion is the higher incidence of adjacent segment disease, which occurs more frequently in proximal segments. Since both coronal deformity and imbalance are corrected by long fusions, the incidence of adjacent segment disease is lower compared to that by short fusions [11]. The present study compared radiologic changes and clinical outcomes of proximal segments postoperatively using VAS pain scores. As has been well established, the risk of adjacent segment problems is substantially higher after a short fusion. In particular, the higher incidence of adjacent segment pathology caused by disc degeneration has been associated with changes in the coronal UIA-1. In order to prevent adjacent segment problems, long fusions extended above the upper segments are recommended rather than a short fusion terminating at segments exhibiting deformity [11]. A previous study [21] reported that the range of fusion depends upon the inclusion of segments with spondylolisthesis, rotatory subluxation, or horizontal vertebra. The range of fusion levels should be based on considerations of the preoperative status and scoliosis severity. In the present study, radiologic and clinical results improved after a short fusion in regions exhibiting deformity, and radiologic progression occurred most frequently between 6 and 12 months after surgery. During follow-up after a short fusion in patients with DLS, radiologic, physical examinations, and the confirmation of radiologic progression are important, especially between 6 and 12 months. The use of additional assistive devices should be considered in patients with greater progression.
In the present study, complications were detected in 5 of 70 patients. A compression fracture in the proximal lumbar spine was identified in one patient with a history of preoperative osteoporosis, and the patient was treated conservatively. The other 4 complications involved a non-union, and 2 patients underwent long fusion revision surgery, due to symptom and condition severities. The other 2 patients who underwent a L3–4–5 fusion experienced scoliotic changes in the lower lumbar spine, as well as an extended fusion to the S1 level.
The present study is limited by a lack of comparison with patients treated using techniques other than a short fusion. Typically, patients who undergo a long fusion have larger Cobb's angles and more severe coronal and sagittal imbalances, whereas a short fusion is associated with less severe preoperative conditions. For this reason, the effect of surgery on clinical and radiologic outcomes may differ from that of DLS. Another limitation of this study is the small number of cases with pseudoarthrosis, which was insufficient to allow any sub-analysis. In addition, this retrospective study is limited by short refractory periods to a short fusion in selected patients and the relatively small sample size.
Conclusions
In this study, a short-segment fusion produced satisfactory clinical and radiological results at 5 years after surgery. However, radiologic variables (Cobb's angle and 1 segment coronal UIAs) should be carefully considered and clinical caution exercised from 6 to 12 months after shortsegment fusion in small DLS (10°–25°).
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.