Neuropathic Pain after Spinal Surgery
Article information
Abstract
Neuropathic pain after spinal surgery, the so-called failed back surgery syndrome (FBSS), is a frequently observed troublesome disease entity. Although medications may be effective to some degree, many patients continue experiencing intolerable pain and functional disability. Only gabapentin has been proven effective in patients with FBSS. No relevant studies regarding manipulation or physiotherapy for FBSS have been published. Spinal cord stimulation (SCS) has been widely investigated as a treatment option for chronic neuropathic pain, including FBSS. SCS was generally accepted to improve chronic back and leg pain, physical function, and sleep quality. Although the cost effectiveness of SCS has been proved in many studies, its routine application is limited considering that it is invasive and is associated with safety issues. Percutaneous epidural adhesiolysis has also shown good clinical outcomes; however, its effects persisted for only a short period. Because none of the current methods provide absolute superiority in terms of clinical outcomes, a multidisciplinary approach is required to manage this complex disease. Further studies concerning the etiology, diagnosis, treatment, and cost effectiveness of FBSS are warranted to deepen our understanding of this condition.
Introduction
Neuropathic pain after spinal surgery is a frequently observed troublesome disease entity for both patients and surgeons [123]. Patients complain of persistent back or leg pain, regardless of the absence of neural compression. Neuropathic pain is frequently not matched with the dermatome and is characterized by its severity and continuity [4]. Although medications may be effective to some degree, many patients continue experiencing intolerable pain and functional disability, leading to psychological disturbances such as depression or insomnia [56]. Because the exact causes of neuropathic pain after spinal surgery have not been identified in many studies, this disease entity has been termed failed back surgery syndrome (FBSS) or post-lumbar surgery syndrome. However, this is a misnomer because it gives an impression of wrong surgery, although no definite evidence is usually found to provoke the symptoms [7]. In addition, many patients with neuropathic pain did not undergo spinal surgery. Only minor traumas, such as a mild traffic accident or falling down, can cause such severe and persistent neuropathic pain. In this respect, neuropathic pain after spinal surgery might be related to some changes in the pain modulation process [8910], which is supported by literature showing the effectiveness of spinal cord stimulation (SCS). Many studies have attempted to reveal the etiology, epidemiology, treatment, and outcomes of FBSS. However, differing definitions and involvements of heterogeneous populations make it difficult to interpret the findings of such studies. With the recent dramatic increase in understanding neuropathic pain, outcomes of various treatments for FBSS have improved. However, none of the treatments completely resolved this complex disease entity. Thus, this study aimed to review the clinical efficacy, adverse effects, and cost effectiveness of each treatment modality for patients with chronic neuropathic pain after spinal surgery.
Etiology and Diagnosis of Neuropathic Pain after Spinal Surgery
Various causes such as residual stenosis, instability, a synovial cyst, a pseudomeningocele, internal disk disruption, epidural fibrosis, facet syndrome, sacroiliac joint syndrome, reflex sympathetic dystrophy, arachnoiditis, and psychological problems have been suggested as possible etiologies of neuropathic pain after spinal surgery [111213]. Determining the exact causes are frequently difficult using magnetic resonance imaging or neurophysiological studies. In addition, sagittal imbalance or back muscle problems can be potential causes of persistent pain [141516]. The difficulties in diagnosis lead to easy failure of any treatment. Revision surgical treatment reportedly showed an inferior efficacy if the structural causes were not preoperatively identified [1718]. Thus, careful history taking, physical examination, and reviews of pre- and postoperative status are considered basic and fundamental criteria for diagnosing FBSS.
The diagnosis of FBSS is somewhat vague and not universal and is considered to include heterogeneous patients who exhibit residual symptoms after spinal surgery. The difficulty in defining this complex disease entity has been well described [19]. However, doctors recognize this characteristic disease, which is supported by one survey study [20]. Although the Douleur Neuropathique en 4 and Leeds Assessment of Neuropathic Symptoms have been suggested as screening tools for neuropathic pain [21], they are less reliable for identifying the neuropathic component of FBSS [22]. This diagnostic ambiguity is an obstacle in the clinical effectiveness of each treatment modality.
Clinical Outcomes of Each Treatment
1. Medication
Only gabapentin has been proven effective in patients with FBSS. In one randomized controlled trial, gabapentin, at a daily maximal dose of 1,800 mg, showed more clinical efficacy than naproxen [23]. Oral gabapentin with an epidural steroid injection showed better results than the injection alone [24]. Pregabalin also has a preventive analgesic effect on postoperative neuropathic pain [25]. Nonsteroidal antiinflammatory drugs have minimal effects on FBSS [23]. The effect of antidepressants has not been reported. Although oral opioid is frequently used to relieve severe pain, its effectiveness in patients with FBSS is limited [26].
2. Exercise, manipulation, and physiotherapy
Only few studies have reported regarding the effect of exercise, manipulation, or physiotherapy on FBSS. A recent study evaluating different exercise programs reported that isokinetic or dynamic lumbar stabilization exercises were more effective than home exercises [27]. No relevant articles have been found regarding the effect of manipulation or physiotherapy on FBSS. Only one retrospective study showed the efficacy of chiropractic management of lumbar spine pain after spinal surgery [28].
3. Spinal cord stimulation
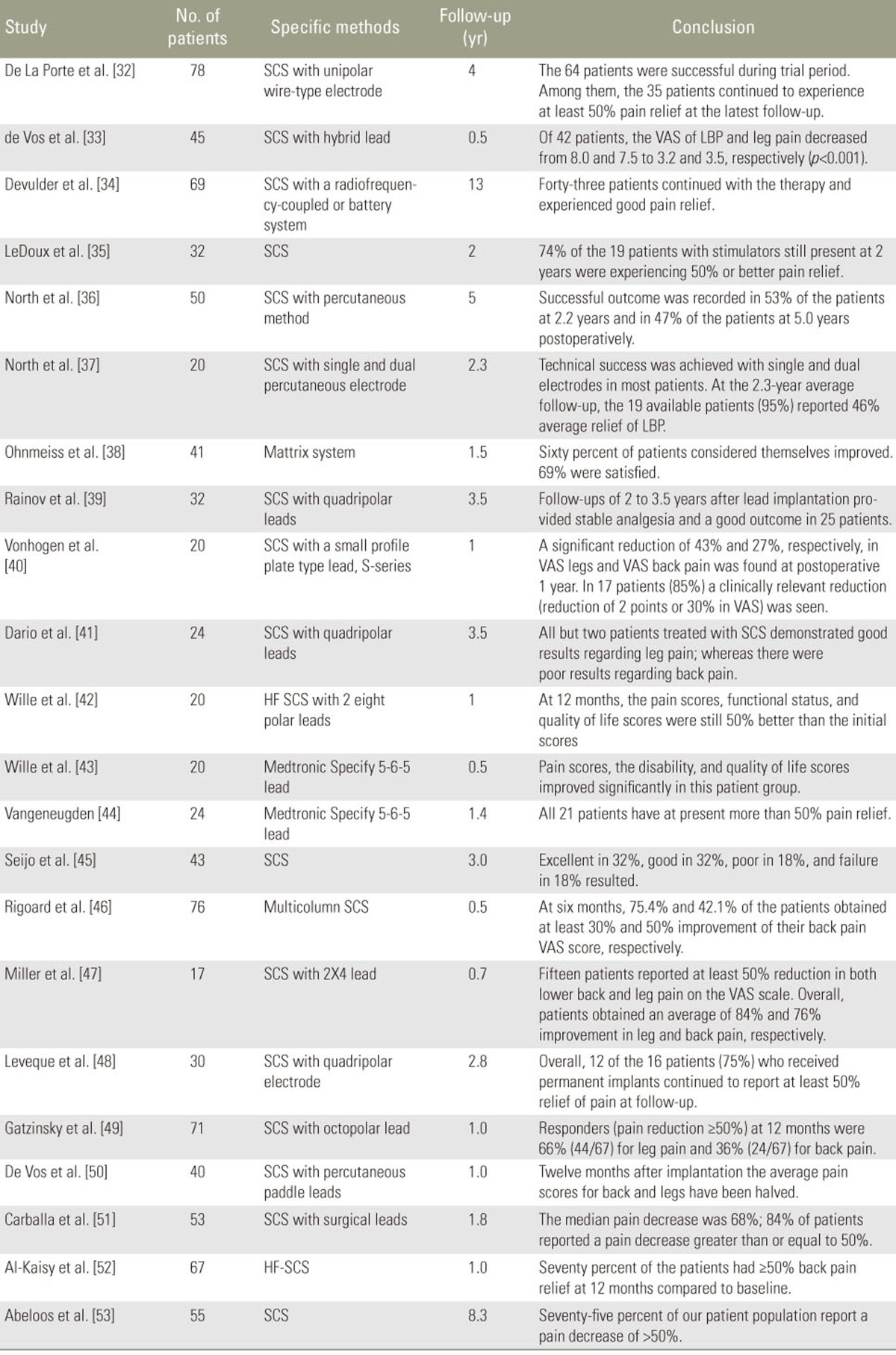
SCS has been widely investigated as a treatment option for chronic neuropathic pain, including FBSS. SCS was generally accepted to improve chronic back and leg pain, physical function, and sleep quality [2930]. The degree of evidence was qualified in one systematic review, which showed level II-1 or II-2 by the U.S. Preventive Services Task Force for long-term relief [31]. Moreover, numerous studies have shown SCS effectiveness (Table 1) [3132333435363738394041424344454647484950515253]. Two randomized controlled trials compared SCS effectiveness with other treatments. A 2-year follow-up study demonstrated the effectiveness of SCS and medication in 48% and 9% of patients, respectively [54]. In the other study, SCS was more effective than repeated spinal surgery (47% vs. 12%, p<0.01) [55]. Regardless of its invasiveness and several complications, SCS is considered to be a relatively safe technique [56].
4. Epidural adhesiolysis
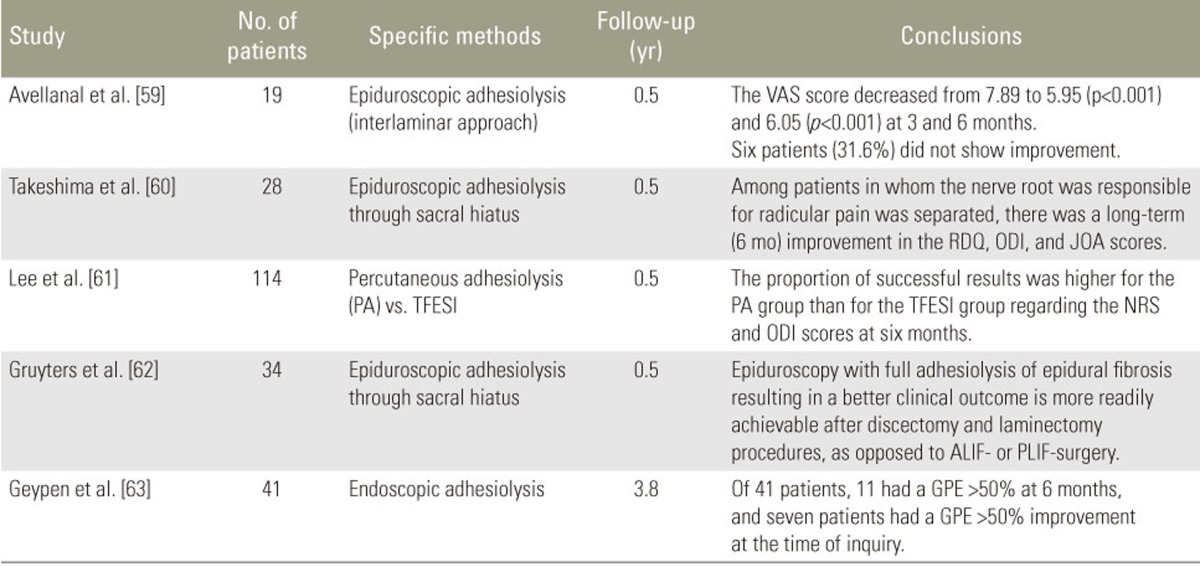
Epidural adhesiolysis releases epidural fibrosis, which could be a source of pain [57]. Manchikanti et al. [58] revealed superior clinical outcomes with epidural adhesiolysis compared with an epidural steroid injection at 1-year follow-up. In addition, several studies showed the short-term (6 months) effectiveness of percutaneous adhesiolysis (Table 2) [5960616263]. These findings were supported by recent systematic reviews [646566]. Complication rates are high in epidural adhesiolysis [67].
5. Injection therapy
Studies that support the effectiveness of injection therapy in FBSS are limited. Although patients who underwent an epidural steroid injection showed immediate improvement, the effect of the therapy deteriorated at 1-, 3-, and 6-month follow-ups [6869]. The differences of clinical outcomes on the basis of injection materials remains unclear [6870].
6. Radiofrequency therapy
There have been few attempts to use radiofrequency to control pain in FBSS. In one study, pulse radiofrequency of the dorsal root ganglion showed good results for three patients during a short-term period [71]. In another study, radiofrequency neurotomy was successful if zygapophysial joint pain existed in patients with FBSS [72].
7. Surgical treatment
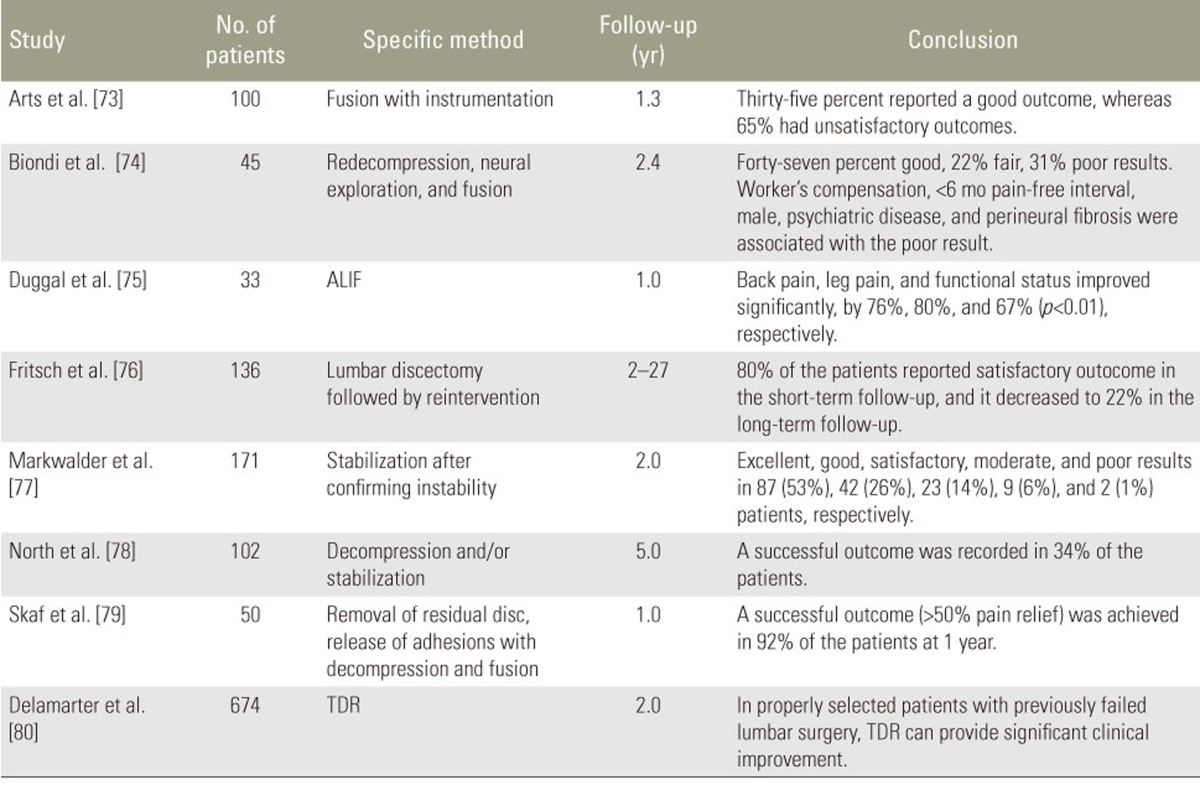
Many studies have revealed the effectiveness of various surgeries, including repeated decompression, instrumented fusion, release of adhesion, or total disk replacement (Table 3) [7374757677787980]. However, varying success rates, ranging from 35% to 92%, have been reported in a short-term (1 year) follow-up. This clinical effect has been significantly aggravated in long-term follow-ups.
Cost Effectiveness
Cost effectiveness is another important consideration for evaluating outcomes per unit, as well as insurance policy recommendations. Because of the considerable economic burden of FBSS, a cost-effectiveness analysis should be performed [81]. Many authors have suggested that SCS is more cost effective than conventional medication or surgery as a treatment of FBSS, regardless of the initial high cost [82838485]. This cost effectiveness is unclear in patients receiving workers' compensation [86]. Overall, the cost-effectiveness analyses of SCS for FBSS are summarized in Table 4 [84858788]. Besides SCS, only one study supported the cost effectiveness of long-term intrathecal morphine therapy [89]. No studies have been found regarding the cost effectiveness of percutaneous epidural adhesiolysis.
Discussion
FBSS is a challenge for surgeons, pain specialists, and primary care providers because of its vague etiology and lack of definite treatment protocols, thereby requiring a multidisciplinary treatment approach [11]. Thorough history taking, physical examination, and careful reviews of imaging are required, followed by the establishment of adequate treatment strategies. Because conventional medical treatments have been considered ineffective, the efficacy, safety, and cost effectiveness of other interventional modalities have been widely investigated.
Among the various interventions, research on SCS has been increasing; SCS is reportedly effective, relatively safe, and cost effective. To relieve pain, SCS is considered to activate dorsal column fibers, which suppress the neuronal activity by activating inhibitory interneurons [90]. Most studies regarding the efficacy of SCS have been retrospective case series, which could contain an intention bias by investigators. Because SCS is an invasive procedure, it is not considered as a primary treatment for FBSS. Complications associated with SCS are not negligible. The most commonly reported complications include hardware malfunction and wound infection [91]. However, fatal complications such as epidural hematoma or spinal cord injury could occur. Nevertheless, as a last resort, a temporary insertion of a cord stimulator can be considered. If it had good clinical outcomes, then a permanent insertion is considered. Several studies suggested an algorithm to manage FBSS with SCS [9293]. The technology of SCS has dramatically developed. The efficacy of SCS may increase by new leads in a multicolumn design and a greater number of contact sites (ESTIMET study) [9495].
Percutaneous epidural adhesiolysis was originally developed to remove the epidural adhesion by surgical intervention, annular tear, or inflammatory response [57]. It reportedly had a strong short-term (3 months) efficacy and moderate long-term (>3 months) efficacy in patients with chronic low back pain [96]. In a recently published systematic review, percutaneous adhesiolysis was efficient in patients with lumbar post-surgery syndrome with level II evidence by Interventional Pain Management Techniques-Quality Appraisal of Reliability and Risk for Bias Assessment [66]. Although weak positive evidence exists regarding epidural adhesiolysis being more effective than epidural steroid injection in FBSS, the complication rates are also higher [67].
Injection therapy can be used as a primary treatment on the basis of its procedure simplicity and rare complications. The various injection procedures include epidural injection (caudal, transforaminal), sympathetic nerve block, intrathecal injection, prolotherapy, and a combination therapy [68979899]. Regardless of the various therapeutic approaches, temporary clinical improvements and lack of a control group are considered to be limitations of studies. More studies are required to reveal the efficacy of radiofrequency therapy.
Surgical management of FBSS showed only limited evidence with variable outcomes, likely caused by the vague definition of FBSS. If a study included surgically correctable cases (i.e., residual stenosis, instability, or disk degeneration), then the clinical outcomes could obviously be improved after careful diagnostic procedures. Therefore, the outcomes of revision surgery for chronic neuropathic pain after index spinal surgery are judged to be ineffective.
Because no single approach has produced excellent results, a multidisciplinary approach is required to manage patients with FBSS. Chronic pain is related to psychological distress after lumbar surgery [100101]. Thus, psychological control is also important. Concurrent rehabilitation by exercise or physiotherapy will be helpful to enhance the clinical effect of other interventions.
Conclusions
FBSS is considered troublesome for both patients and doctors because it usually does not respond to conventional medication or injection therapy. Although the literature reports relatively good clinical outcomes and cost effectiveness with the use of SCS, its routine application should be limited considering that it is invasive and is associated with safety issues. Meanwhile, although epidural adhesiolysis has shown good clinical outcomes, its effects persisted for only a short period. A multidisciplinary treatment approach is required to manage this complex disease entity because none of the current methods provide absolute superiority. Further studies regarding the etiology, diagnosis, treatment, and cost effectiveness of FBSS are warranted to deepen our understanding of the condition.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.