Comparison of Clinical and Radiological Outcomes of Lumbar Interbody Fusion Using a Combination of Hydroxyapatite and Demineralized Bone Matrix and Autografts for Lumbar Degenerative Spondylolisthesis
Article information
Abstract
Study Design
Prospective, cohort, non-inferiority study.
Purpose
This study evaluated the clinical and radiological outcomes of interbody fusion using a combination of demineralized bone matrix (DBM) and hydroxyapatite (HA).
Overview of Literature
The use of autografts remains a gold standard in lumbar interbody fusion, but the limited availability and donor site morbidity encourages the use of bone substitutes. In addition to autografts, a combination of HA and DBM is being increasingly use for lumbar interbody fusion. However, there are no data on the clinical and radiological outcomes of this procedure.
Methods
We examined 35 patients with lumbar degenerative spondylolisthesis who underwent transforaminal interbody fusion. Autografts were used in 18 patients, and 17 patients received a combination of HA and DBM. Clinical outcomes were evaluated using the visual analog scale (VAS) for back and leg pain, Oswestry disability index (ODI), and Japanese Orthopaedic Association (JOA) scores at 3, 6, and 12 months postoperatively. Fusion was evaluated using computed tomography images obtained at 12 months postoperatively.
Results
The mean ODI, JOA, and back and leg pain VAS scores increased significantly in both groups. However, the VAS, JOA, and ODI scores did not differ significantly between the two groups (p=0.599, p=0.543, and p=0.780, respectively). The fusion rates at 1 year postoperatively were 77.8% and 76.5% in the autograft and HA+DBM groups, respectively (p=0.99).
Conclusions
The clinical and radiological outcomes of using a combination of HA and DBM in lumbar interbody fusion were not inferior to those of using autografts. A combination of HA and DBM can be considered as an alternative in patients with lumbar degenerative spondylolisthesis requiring surgery.
Introduction
Considering that pain can be relieved with good arthrodesis, lumbar fusion is one of the most commonly used procedures in patients with lumbar degenerative spondylolisthesis [12]. Several studies have shown that the clinical outcomes of this procedure are usually associated with good bone fusion between two vertebrae [345]. For achieving fusion, autografts are still the gold standard owing to their potential for osteoconductivity, osteoinductivity, and osteogenicity [6]. Despite their high potential, autografts have limitations such as limited availability and donor site morbidity [3]. Furthermore, disruption in the performance of daily activities and discomfort while walking have been reported [7].
To overcome the limitations of autografts, the use of bone substitutes has increased, although there are no substitutes having properties similar to autografts [8]. A combination of several bone substitutes has been used mainly for achieving spinal fusion. Demineralized bone matrix (DBM) is an allogenic bone substitute comprising bone morphogenic protein, collagen, protein, and growth factors such as transforming growth factor and insulin growth factor [9]. Therefore, it has both osteoinductive and osteoconductive capacities and can promote bone healing through growth factors, which initiate mesenchymal cell differentiation into osteoblasts [6]. Hydroxyapatite (HA) is frequently used as an autograft extender and has osteoconductive properties. A previous study has shown comparable result to autograft [3].
Several animal studies have compared a combination of DBM and HA with autografts and demonstrated comparable results [10]. Research conducted by Tilkeridis et al. [11] on posterolateral fusion revealed no statistically significant differences between autografts and DBM. Miyazaki et al. [8] stated that DBM exhibited a 75% fusion rate. The use of different DBM brands has also exhibited variable results. This study evaluated the clinical and radiological outcomes of using a combination of DBM and HA for interbody fusion.
Materials and Methods
We conducted a prospective, cohort, non-inferiority study of patients with lumbar degenerative spondylolisthesis who underwent transforaminal interbody fusion (TLIF) between July 2014 and January 2015. The patients who underwent TLIF using autografts or a combination of DBM and HA were followed up. The DBM used in this study was from Bongener (Daewoong, Seoul, Korea) and HA was from Bongros-HA (Daewoong). Bongener (Daewoong) is a combination of DBM and a blood-controlled thermoresponsive polymer with effective osteoinductive properties. Bongros-HA (Daewoong) is a type of ceramic or bone-chip synthetic material made of porous HA (Ca10(PO4)6(OH)2), which is usually used as a bone graft extender; the particle size ranges from 3.0 to 6.0 mm. In our 6-month study period, 65 patients fulfilled the inclusion criteria but 10 patients refused participation. Of the remaining 55 patients, 27 and 28 patients were operated using autografts and a combination of DBM and HA, respectively. In each group, nine patients were lost to follow-up at 1 year. Patient characteristics in both groups exhibited similar age and sex distributions, operation levels, and body mass indices (Table 1).
1. Surgical procedures
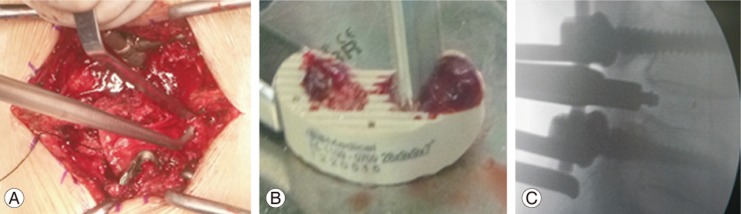
All patients underwent single-level TLIF, which was performed in a standard manner as previously reported [12]. The polyetheretherketone cages were filled with either an autograft or a combination of DBM and HA (Fig. 1). Foraminectomy was conducted on the side with most severe clinical signs and symptoms.
2. Clinical and radiological outcomes
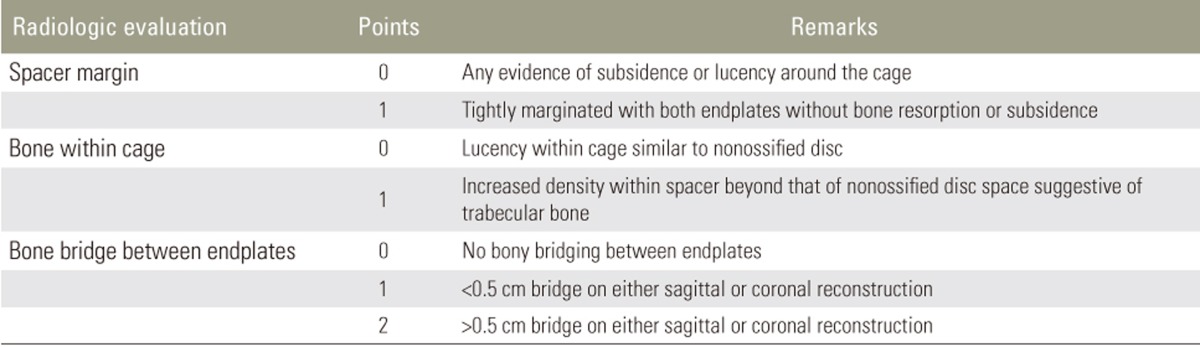
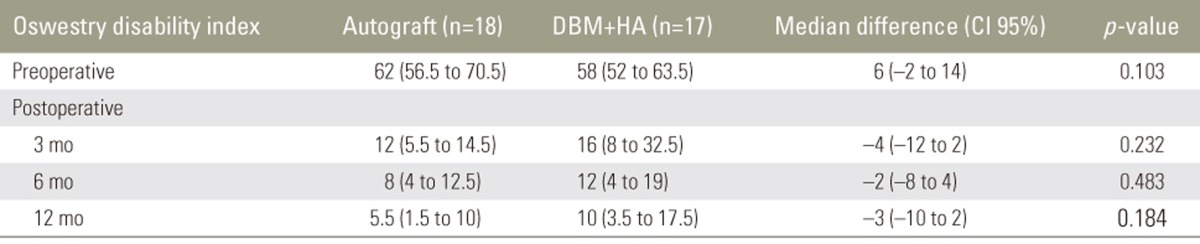
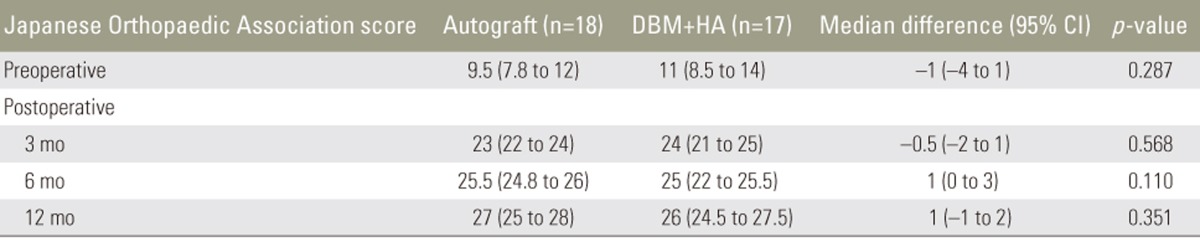
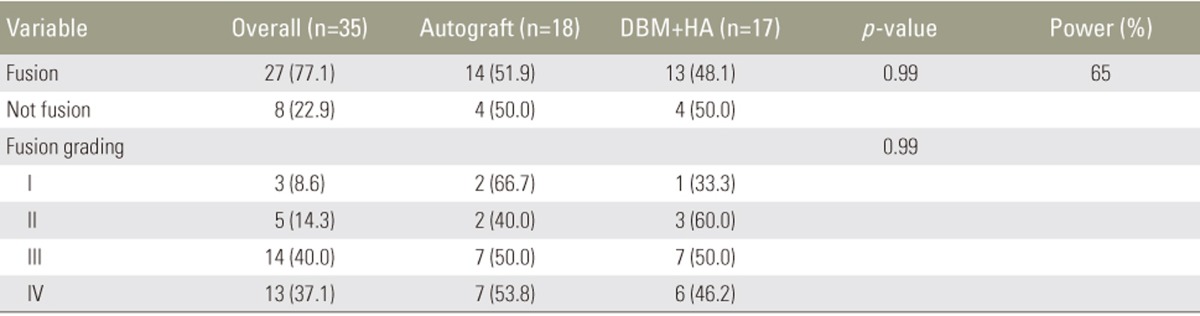
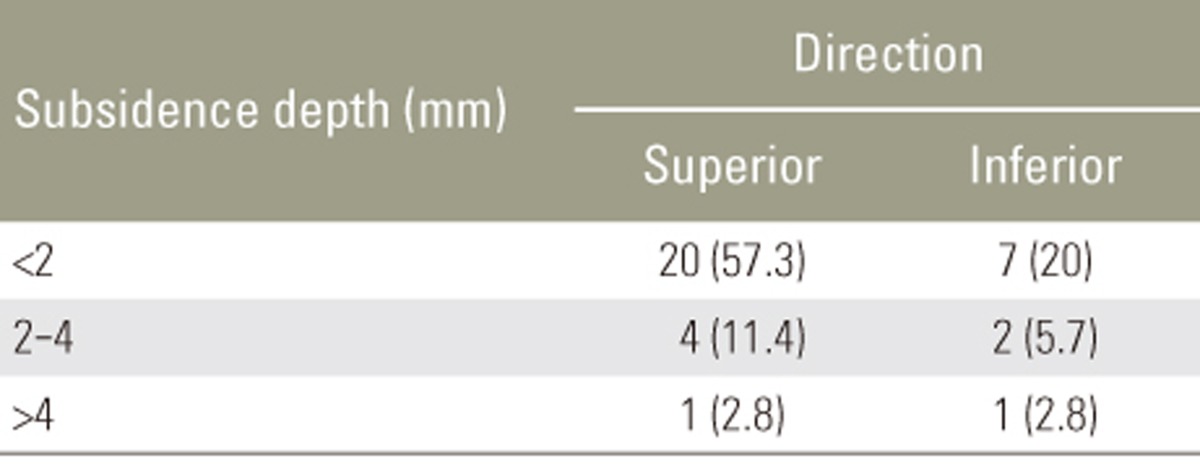
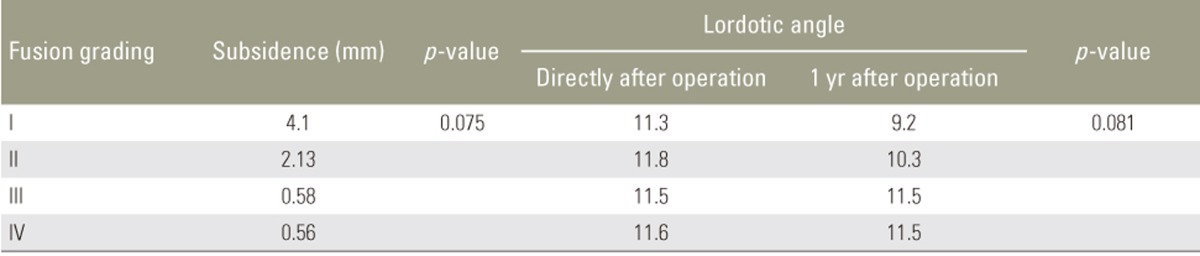
Clinical outcomes were evaluated using the visual analog scale (VAS) for back and leg pain, the Oswestry disability index (ODI), and the Japanese Orthopaedic Association (JOA) score. All the outcomes were assessed preoperatively and at 3, 6, and 12 months postoperatively. The fusion rate was determined using the criteria reported by Girasole et al. [12], which was applied by a musculoskeletal radiologist (Table 2). Fusion was indicated by a score of 3 or 4. The maintenance of fusion sites was evaluated based on the occurrence of subsidence and the lordotic angles at 1 year postoperatively.
3. Statistical analysis
All research data were evaluated using SPSS ver. 17 (SPSS Inc., Chicago, IL, USA). Numerical data were analyzed using the Mann-Whitney U (non-parametric statistic) test, and proportion data were analyzed using the Fisher exact test.
4. Ethical approval
Ethical approval for this research was provided by the Ethical Research Committee of Ciptomangunkusumo National Reference Hospital, Faculty of Medicine, Universitas Indonesia.
Results
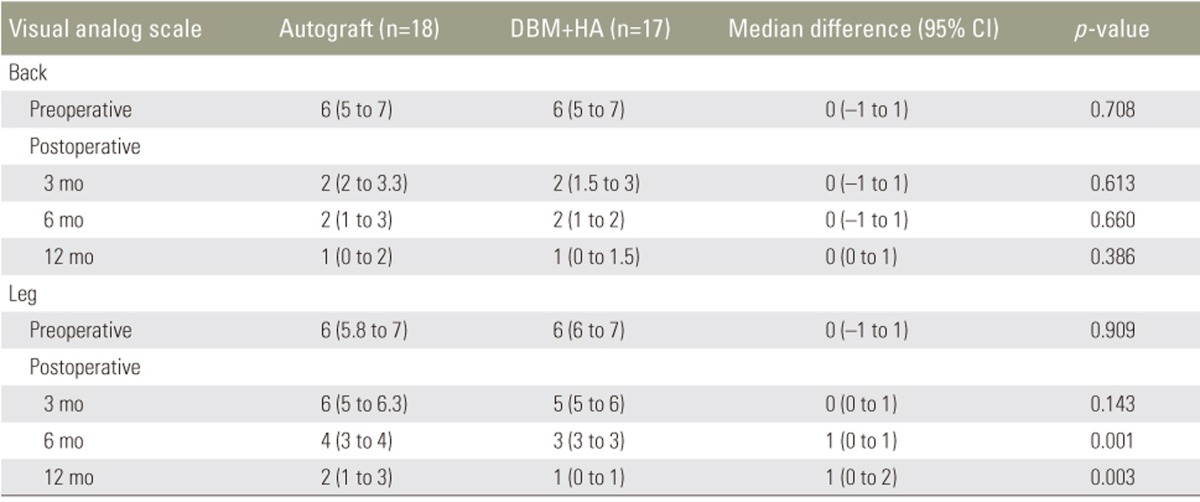
Overall, 35 patients were followed up until 1 year. The median preoperative back and leg pain VAS score in the autograft and combination groups was 6 (5–7) out of 10, and the scores in both groups showed a decreasing trend on follow-up. At 3, 6, and 12 months postoperatively, the back VAS scores were 2 (2–3.3), 2 (1–3), and 1 (0–2) in the autograft group and 2 (1.5–3), 2 (1–2), and 1 in the combination group, respectively (all p>0.05). The median leg pain VAS scores at 3, 6, and 12 months postoperatively were 6 (5–6.3), 4 (3–4), and 2 (1–3) in the autograft group and 5 (5–7), 3 (3–3), and 1 (0–1) in the combination group, respectively. The leg pain VAS scores showed significant differences between the two groups at 6 and 12 months postoperatively (p=0.001 and p=0.003, respectively) (Table 3).
The median preoperative ODI score was 62 (56.5–70.5) in the autograft group and 58 (52–63.5) in the combination group; on follow-up, the scores showed significant improvement in both groups. In the autograft group, the scores at 3, 6, and 12 months postoperatively were 12 (5.5–14.5), 8 (4–12.5), and 5.5 (1.5–10) in the autograft group and 16 (8–32.5), 12 (4–19), and 10 (3.5–17.5) in the combination group, respectively. There were no significant differences between the two groups (Table 4).
The JOA score did not significantly differ between the autograft and combination groups. Both groups showed significant improvement in the scores until 12 months postoperatively. The scores increased from 9.5 (7.8–12) preoperatively to 27 (25–28) at 12 months postoperatively in the autograft group and from 11 (8.5–14) to 26 (24.5–27.5) in the combination group (Table 5).
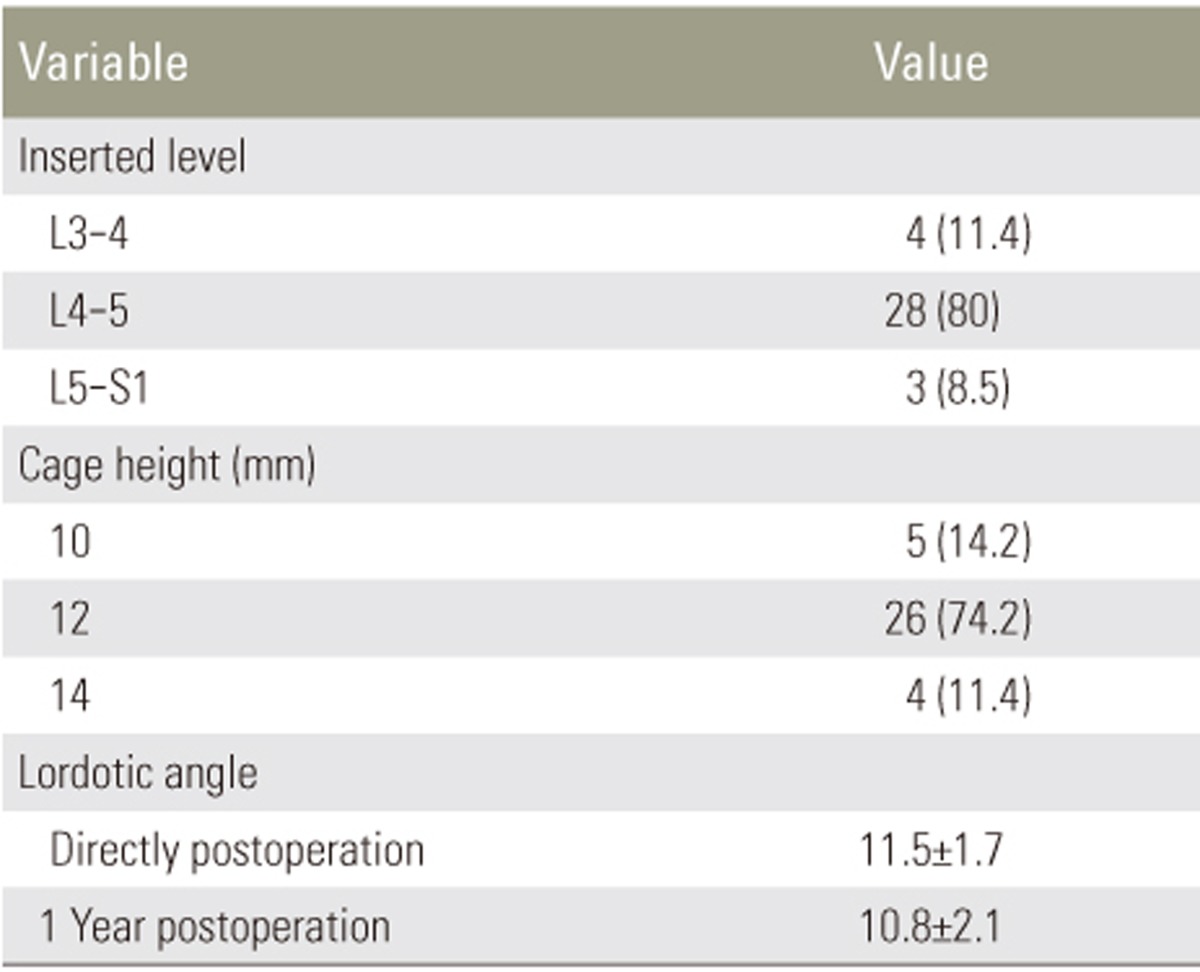
The fusion rates at 1 year were 77.8% in the autograft group and 76.5% in the combination group (p=0.99). Among the study population, 8.6% exhibited a fusion grade I; 14.3%, grade II; 40%, grade III; and 37.1%, grade IV. Among patients with fusion grades III and IV, solid bony bridging between two vertebral bodies in both the autograft and combination groups (Table 6), minimal subsidence of <2 mm, and maintenance of the lordotic angle at 1 year postoperatively (p<0.05) were observed. In the non-fusion group, subsidence of >4 mm and loss of the lordotic angle were observed, indicating fixation failure, which results in sagittal imbalance (p<0.05) (Tables 7, 8, 9).
Discussion
Lumbar interbody fusion surgery is a procedure for the management of lumbar degenerative spondylolisthesis, and this procedure can be performed with or without instrumentation [1]. The goal is to achieve solid bony fusion between vertebral bodies. Autografts from the iliac crest were previously the gold standard; however, several studies are recently evaluating the use of local bone chips obtained from laminectomy [1314]. Hashimoto et al. [15], Ido et al. [16] and Miura et al. [17] and have reported that local bone chips had fusion rates of approximately 70%, which is lower than the fusion rate (85%–95%) achieved using iliac crest autografts [15161718].
Several studies on bone substitutes have been conducted. Khan et al. [13] and Grabowski and Cornett [4] compared the use of autografts, autograft+DBM, and DBM alone in posterolateral fusion and reported no significant differences. Using animal models, Smucker and Fredericks [19] and Lee et al. [20] demonstrated similar histologically mature bone formation in both autograft and DBM groups after posterolateral fusion. In South Korea, Lee et al. [21] used DBM in direct lateral lumbar interbody fusion and reported a 60.9% fusion rate at 6 months and 87.5% at 12 months postoperatively. In this study, computed tomography images obtained at 12 months postoperatively revealed a fusion rate of 76.5% in the DBM+HA group, with bony bridging that appeared to be solid in coronal and sagittal views (Fig. 2). Two patients from the DBM+HA group and one from the autograft group were diagnosed with non-union, but the remaining patients in the non-fusion group were still followed up owing to signs of slow fusion progression (Fig. 3).

Computed tomography images obtained at 12 months postoperatively in the demineralized bone matrix+hydroxyapatite group. Sagittal view (A) and coronal view (B); both images show solid bony bridging between two vertebral bodies.
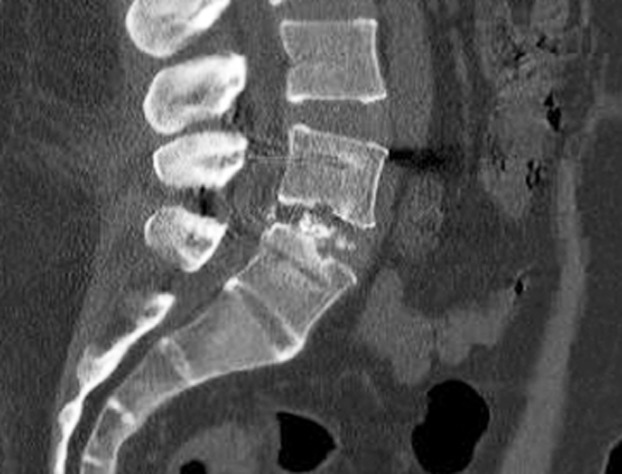
Sagittal computed tomography images obtained at 12 months postoperatively in the demineralized bone matrix+hydroxyapatite group. Image shows non-union at 12 months postoperatively. The patient underwent revision surgery.
Clinical outcomes of lumbar degenerative spondylolisthesis are dependent on the fusion rate. Kasliwal and Deutsch [1] showed a decrease in VAS scores of >50% at 14 months postoperatively. Lee et al. [18] also reported good postoperative ODI and VAS scores compared with preoperative scores. In our study, the postoperative VAS scores for back pain decreased significantly in both groups; however, the scores indicated residual leg pain in the autograft group (Figs. 4, 5). According to Hunt et al. [22] residual leg pain can be caused by excessive dural retraction, mobilization of the dura mater, and distraction on the decompression side, which can lead to compression of the contralateral foramen and excessive lumbar lordosis correction [2324].
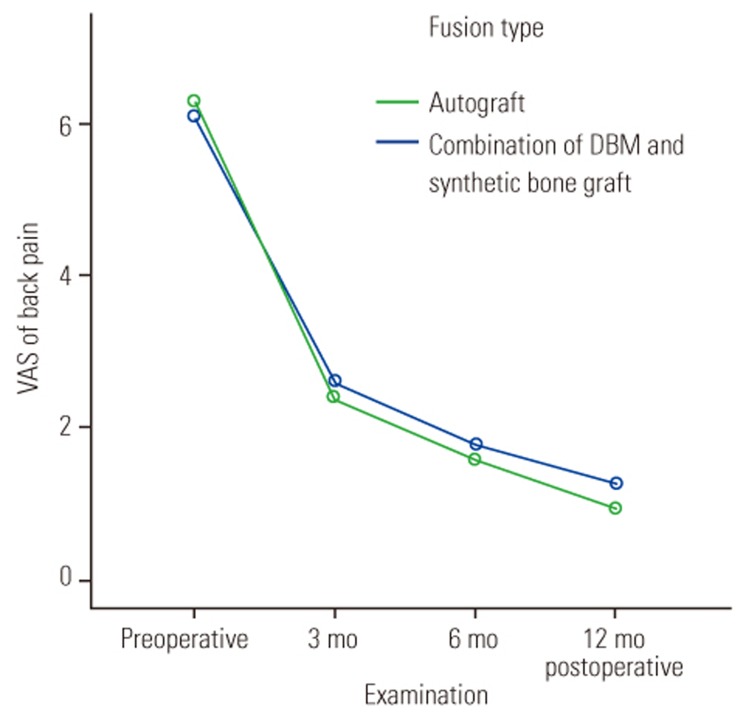
Comparison of back pain visual analog scale (VAS) scores between the autograft and demineralized bone matrix (DBM)+hydroxyapatite groups.
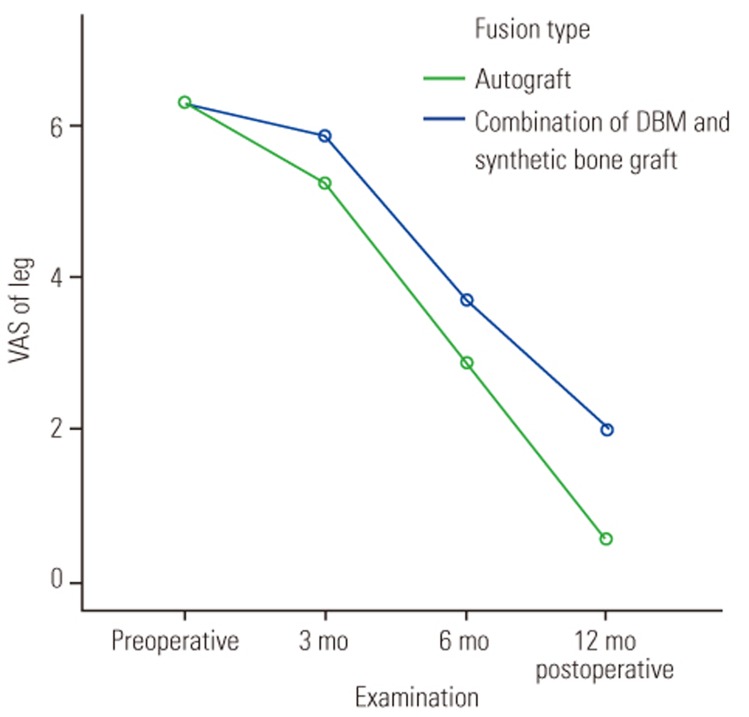
Comparison of leg pain visual analog scale (VAS) scores between the autograft and demineralized bone matrix (DBM)+hydroxyapatite groups.
In this study, the JOA score showed a 50%–60% increase in the fusion (autograft or combination) group, whereas in the non-fusion group, only an increase of 20%–30% was observed (Fig. 6). Significant differences between the fusion and non-fusion groups were also detected. The ODI scores were comparable between the autograft and DBM+HA groups; however, in the non-fusion group, moderate disability was noted at 12 months (Fig. 7). This result is in accordance with that of a previous study showing that an increase in ODI scores is parallel to the extent of fusion and patient satisfaction [25]. Yu et al. [26] stated that good fusion indicates good clinical outcomes in lumbar spondylosis. In addition, Zigler and Delamarter [27] reported that after fusion, patients showed improved quality of life and ability to perform recreational activities.

Comparison of Japanese Orthopaedic Association scores between the autograft and demineralized bone matrix (DBM)+hydroxyapatite groups.

Comparison of Oswestry disability index scores between the autograft and demineralized bone matrix (DBM)+hydroxyapatite groups.
This study had some limitations, including a limited number of patients and a 12-month follow-up period, which might be too short to precisely evaluate the outcome. Other parameters for evaluating fusion, such as cage subsidence and lumbar lordosis, were not assessed in this study.
Conclusions
In conclusion, the use of a combination of DBM and HA exhibited clinical and radiological outcomes comparable to those obtained using autografts. Despite being inferior to autografts, bone substitutes comprising DBM+HA can be considered as an alternative to autografts. We detected no significant differences between the autograft and combination groups.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.