|
 |
- Search
| Asian Spine J > Volume 11(1); 2017 > Article |
|
Abstract
Purpose
This study was to investigate interside asymmetries of three lower extremity somatosensory evoked potentials (SSEPs) in anesthetized patients with unilateral lumbosacral radiculopathy.
Overview of Literature
Although interside asymmetry is an established criterion of abnormal SSEP, little is known which of the lower SSEPs is more sensitive in detecting interside asymmetry in anesthetized patients.
Methods
Superficial peroneal nerve SSEP (SPN-SSEP), posterior tibial nerve SSEP (PTN-SSEP), and sural nerve SSEP were obtained in 31 lumbosacral surgery patients with unilateral lumbosacral radiculopathy, and compared with a group of 22 control subjects.
Results
The lumbosacral group showed significant larger interside asymmetry ratios of P37 latencies in SPN-SSEP and PTN-SSEP, and significant larger interside asymmetry ratio of P37-N45 amplitude in SPN-SSEP, when comparing with the control group. Within the lumbosacral group but not the control group, SPN-SSEP displayed significant larger interside asymmetry ratio in P37 latency. When referencing to the control group, more patients in the lumbosacral group displayed abnormal interside SPN-SSEP latency asymmetries
which corroborated the symptom laterality.
Interside asymmetry significantly beyond the control level is an important criterion of abnormal somatosensory evoked potential (SSEP) [12]. In theory, this parameter can be easily applied to anesthetized patients as the within-subject ratio is less likely subject to anesthetic influence.
For decades, post-tibial nerve SSEP (PTN-SSEP) has proven to be well suited for central nervous system evaluation [3]. However, it is still unclear of its adequacy in detecting lumbar nerve root injuries owing to the multi-root (L4ŌĆōS3) inputs [45]. Superficial peroneal nerve and sural nerve mainly represent L4ŌĆō5 and S1ŌĆō2 dorsal roots, respectively [6]; these cutaneous nerve derived SSEPs have been successfully used in anesthetized patients with signal-to-noise ratios comparable to PTN-SSEP [78].
This study is to determine which of the lower SSEPs is more sensitive in detecting interside asymmetry in anesthetized patients by a comparison between PTN-SSEP, superficial peroneal nerve SSEP (SPN-SSEP), and sural nerve SSEP (SN-SSEP).
Lower extremity SSEPs were obtained from both lumbosacral and control patient groups. In the lumbosacral patient group, surgeries were performed in 31 patients who were unilateral symptomatic (mean age┬▒standard deviation, 54.9┬▒15.1; 19 females and 12 males). Of the 31 lumbosacral patients, sixteen were left symptomatic; 15 right symptomatic. Seventeen surgeries were performed at or above the L5 vertebral level; 14 surgeries involved the S1 and adjacent vertebral levels. Determination of the surgical level(s) was based on the patient's symptoms, neurological examination and medical imaging findings. The control group consisted of 22 patients (mean age┬▒standard deviation, 54.4┬▒8.0; 13 women and 9 men) who underwent anterior cervical discectomy and fusion. Clinical pictures of the control group were confined to cervical region without lumbosacral symptoms. All the surgical procedures were performed in a single facility under identical total intravenous anesthesia (TIVA) protocol. This study was approved by the Institutional Review Board and the informed consent was obtained from each patient.
Cortical SSEP waveforms derived from CzŌĆÖ and CiŌĆÖ montages were obtained by electrical stimulation of superficial peroneal, posterior tibial, and sural nerves at the ankle using a Cadwell Cascade (Cadwell Laboratories, Inc., Kennewick, WA). Specifically, the cathodes (stimulating electrodes) were placed (1) behind the medial malleolus for PTN-SSEP; (2) medial to the peroneus tertius at the level of lateral malleolus for SPN-SSEP; and (3) behind the lateral malleolus for SN-SSEP, respectively. Bilateral interleaving stimulation was sequentially presented to the three stimulus sites at intensity about 40 mA. The subdermal EEG electrodes were positioned at CzŌĆÖ-Fpz, CiŌĆÖ-CcŌĆÖ for the cortical P37/N45 and at Fpz-C5Sp for subcortical P31/N34 recordings according to the international 10ŌĆō20 standard. Electrode impedance was kept below five k╬® with inter-electrode balance within two k╬®. A total of 200 averages for each trial, filter bandpass of 30ŌĆō1,000 Hz, and 100 milliseconds analysis epoch were used. Cortical SSEP peak (P37) latencies were measured from the offset of evoked stimulus; amplitudes were measured as the maximal magnitude of separation between peak and trough of P37-N45 complex. SSEPs were recorded after incision was completed but before the microscope was brought to the surgical field. To calculate the interside asymmetry ratios of P37 latency and P37-N45 amplitude, following equation was used:|(LiftŌĆōRight)|/(Left+Right).
The differences of interside asymmetry of SSEP P37 latencies and P37-N45 amplitudes between the lumbosacral and control groups were analyzed by independent t tests; whereas the interside asymmetries between SPN-, PTN-, and SN-SSEPs P37 latencies and P37-N45 amplitudes within either group were analyzed by repeated measures analysis of variance and post-hoc LSD pairwise comparison. Prolongation of interside latency or reduction of interside amplitude ratios beyond 2.5 standard deviations greater than the mean of control group was considered abnormal [1]. All statistical analyses were performed with SPSS ver. 19.0 (SPSS Inc., Chicago, IL, USA). Differences were considered significant when p<0.05.
The interside asymmetry ratios of P37 latencies and P37-N45 amplitudes of SPN-, PTN-, and SN-SSEP were compared between the lumbosacral and control groups (Fig. 1). Significant difference in the interside asymmetry ratios of P37 latency between the groups were found in SPN-SSEP (t=2.105, p=0.04) and PTN-SSEP (t=2.079, p=0.043) (Fig. 1A). In addition, significant difference in the interside asymmetry ratio of P37-N45 amplitude between the groups were found in SPN-SSEP (t=2.142, p=0.037) (Fig. 1B). In contrast, SN-SSEP showed no significant difference in the interside asymmetry ratio between the groups.
The interside asymmetry ratios of P37 latency and P37-N45 amplitude were analyzed between SPN-, PTN- and SN-SSEP within either group (Fig. 2). In the lumbosacral group, significant differences in the interside asymmetry ratio of P37 latency were observed between SPN|PTN and between SPN|SN (SPN vs. PTN, p=0.026; SPN vs. SN, p=0.006) (Fig. 2A). No such differences were found in the control group (Fig. 2B). In addition, there was no significant difference in the interside asymmetry ratios of P37-N45 amplitude between all the SSEPs in either the lumbosacral or control group (Fig. 2C, D).
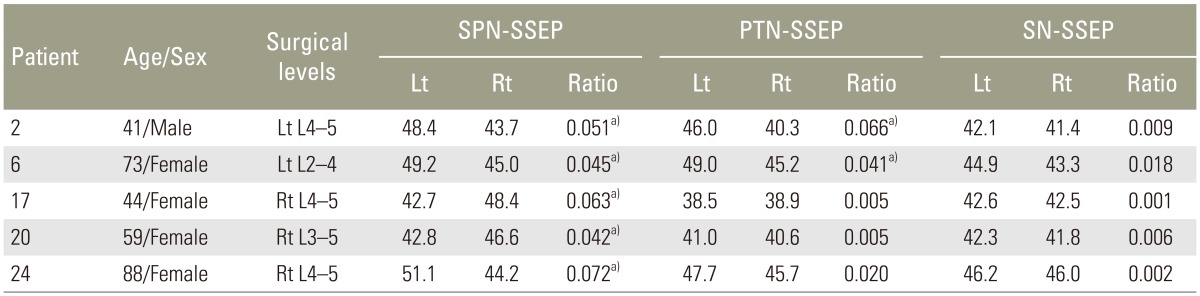
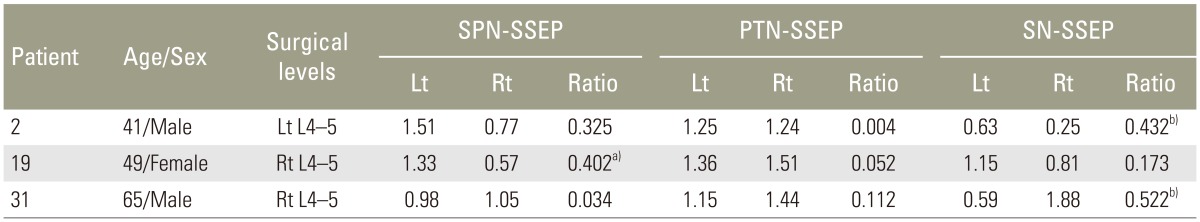
When referencing to the normative values of interside latency (mean+2.5SD) and amplitude (meanŌĆō2.5SD) ratios derived from the control group, the patients with abnormal interside asymmetry ratios of P37 latency (Table 1) and P37-N45 amplitude (Table 2) were shown. A higher incidence of the abnormal latency asymmetry ratio was noted in SPN-SSEP (5/31, ~16%), followed by PTN-SSEP (2/31, ~6%). All the abnormal latency asymmetry ratios matched the symptom sides.
As more advanced diagnostic tools have become available to aid the surgical decisions nowadays, SSEP is less frequently administered as part of the preoperative workups [4]. Nevertheless, studies had indicated that SSEP abnormalities strongly suggested a true compromise of the corresponding nerve root(s) in patients with radiculopathy, whereas the structural lesion on an imaging study did not always translate into abnormal axonal conductivity [910]. As such, our center uses the asymmetric SSEP as a surrogate marker of hidden culprits causing the root compression when the initial operative findings do not match the degree of SSEP abnormalities.
In lumbosacral patients, spondylotic radicular symptoms are often caused by L4 and L5 foraminal narrowing [910]. The higher degree and incidence of SPN-SSEP latency asymmetry was likely associated with its L4 and L5 origins. PTN-SSEP did not match the sensitive level of SPN-SSEP (PTN-SSEP ~6% vs. SPN-SSEP ~16%); it merely confirmed the PTN-SSEP findings. SN-SSEP showed the least amount of latency asymmetry, presumably due to the low incidence of S1 and S2 foraminal stenosis. Although S1 radiculopathy is common, its involvement is often bilateral due to the sacral fibers are more medially situated in the cauda equine and subject to midline compression [11]. The midline compression to bilateral S1 roots is unlikely to cause a notable interside difference in SN-SSEP.
In the patients with isolated compressive lumbosacral root lesions, the mixed nerve SSEP was almost always normal because of the multi-segment property of the mixed nerves [4]. To evaluate individual root function, techniques such as dermatomal somatosensory evoked potential (DSSEP) were developed to restrict SSEP input in a single nerve root. Although some reported that DSSEP was a sensitive measure for lumbosacral radiculopathies [12], other found DSSEP was rarely helpful in diagnosis [13]. The low signal-to-noise ratio rendered the on-line interpretation of DSSEP waveform difficult in the operating theater [14].
SSEPs elicited by cutaneous nerve stimulation are more segmentally specific than those from the mixed nerve stimulation, yet the waveforms are usually robust and consistent [4]. This study found SPN-SSEP was more sensitive in detecting unilateral compressive lesions than PTN-SSEP in anesthetized patients, which was largely in agreement with the earlier findings from non-anesthetized subjects [1015161718]. In this study all SSEP recordings were performed under identical TIVA anesthesia protocol. Caution should be exercised when using inhalation gases, as the level of cortical suppression was a known factor altering interside interpeak latency difference [19].
The present study found SSEP latency asymmetries corroborated the clinical symptoms, suggesting latency asymmetry might be a good indicator in quantifying conduction delay in the anesthetic state. On the other hand, the incidence of abnormal interside amplitude asymmetries was low; when it did occur, the asymmetry preponderance did not always match the symptom side. Isolated abnormalities of SSEP amplitude should be interpreted with cautions, especially without concomitant latency delay [1].
The lower incidence of interside amplitude asymmetry in lumbosacral patients was presumably a result of central synaptic amplification which had, at least partially, compensated the nerve root axonal loss in the disease process [20]. This is in contrast to the acute laceration of nerve fibers during surgery, which may show an isolated attenuation of SSEP amplitude without concomitant latency delay [21].
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
1. American Clinical Neurophysiology Society. Guideline 9D: guidelines on short-latency somatosensory evoked potentials. J Clin Neurophysiol 2006 23:168ŌĆō179. PMID: 16612233.


2. Moncho D, Poca MA, Minoves T, Ferre A, Sahuquillo J. Interside latency differences in brainstem auditory andsomatosensory evoked potentials: defining upper limits to determine asymmetry. J Clin Neurophysiol 2015 32:424ŌĆō427. PMID: 26061480.


3. Nuwer MR, Dawson EG, Carlson LG, Kanim LE, Sherman JE. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol 1995 96:6ŌĆō11. PMID: 7530190.


4. Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. American Association of Electrodiagnostic Medicine. Muscle Nerve 1998 21:1612ŌĆō1631. PMID: 9843062.


5. Tsai SW, Tsai CL, Wu PT, Wu CY, Liu CL, Jou IM. Intraoperative use of somatosensory-evoked potential in monitoring nerve roots. J Clin Neurophysiol 2012 29:110ŌĆō117. PMID: 22469674.


6. Chiappa KH. Evoked potentials in clinical medicine. 3rd ed. Philadelphia: Lippinocott-Raven; 1997.
7. Yue Q. P37 latency mismatch between lateral and midline potentials is influenced by transversal afference. J Clin Neurophysiol 2015 32:30ŌĆō33. PMID: 25502101.


8. Pelosi L, Caruso G, Cracco RQ, Cracco JB, Balbi P. Intraoperative recordings of spinal somatosensory evoked potentials to tibial nerve and sural nerve stimulation. Muscle Nerve 1991 14:253ŌĆō258. PMID: 2041546.


9. Perlik S, Fisher MA, Patel DV, Slack C. On the usefulness of somatosensory evoked responses for the evaluation of lower back pain. Arch Neurol 1986 43:907ŌĆō913. PMID: 2943255.


10. Dumitru D, Dreyfuss P. Dermatomal/segmental somatosensory evoked potential evaluation of L5/S1 unilateral/unilevel radiculopathies. Muscle Nerve 1996 19:442ŌĆō449. PMID: 8622722.


11. Wall EJ, Cohen MS, Massie JB, Rydevik B, Garfin SR. Cauda equina anatomy I: Intrathecal nerve root organization. Spine (Phila Pa 1976) 1990 15:1244ŌĆō1247. PMID: 2281366.


12. Katifi HA, Sedgwick EM. Evaluation of the dermatomal somatosensory evoked potential in the diagnosis of lumbo-sacral root compression. J Neurol Neurosurg Psychiatry 1987 50:1204ŌĆō1210. PMID: 3668570.



13. Aminoff MJ, Goodin DS, Barbaro NM, Weinstein PR, Rosenblum ML. Dermatomal somatosensory evoked potentials in unilateral lumbosacral radiculopathy. Ann Neurol 1985 17:171ŌĆō176. PMID: 2983601.


14. Tsai RY, Yang RS, Nuwer MR, Kanim LE, Delamarter RB, Dawson EG. Intraoperative dermatomal evoked potential monitoring fails to predict outcome from lumbar decompression surgery. Spine (Phila Pa 1976) 1997 22:1970ŌĆō1975. PMID: 9306525.


15. Eisen A, Hoirch M, Moll A. Evaluation of radiculopathies by segmental stimulation and somatosensory evoked potentials. Can J Neurol Sci 1983 10:178ŌĆō182. PMID: 6311388.


16. Walk D, Fisher MA, Doundoulakis SH, Hemmati M. Somatosensory evoked potentials in the evaluation of lumbosacral radiculopathy. Neurology 1992 42:1197ŌĆō1202. PMID: 1318522.


17. Seyal M, Sandhu LS, Mack YP. Spinal segmental somatosensory evoked potentials in lumbosacral radiculopathies. Neurology 1989 39:801ŌĆō805. PMID: 2657484.


18. Tans RJ, Vredeveld JW. Somatosensory evoked potentials (cutaneous nerve stimulation) and electromyography in lumbosacral radiculopathy. Clin Neurol Neurosurg 1992 94:15ŌĆō17. PMID: 1321692.


19. Emerson RG, Sgro JA, Pedley TA, Hauser WA. State-dependent changes in the N20 component of the median nerve somatosensory evoked potential. Neurology 1988 38:64ŌĆō68. PMID: 3336466.


20. Daube JR. Clinical neurophysiology. 2nd ed. New York: Oxford University Press; 2002.
21. Yamada T, Tucker M, Husain AM,Spinal cord surgery. Husain AM, editors. A practical approach to neurophysiologic intraoperative monitoring. New York: Demos Medical Publishing, LLC; 2008. p.117ŌĆō137.
Fig.┬Ā1
Independent t test analysis of the interside asymmetry ratio of P37 latencies (A) and P37-N45 amplitudes (B) of superficial peroneal nerve (SPN)ŌĆō, posterior tibial nerve (PTN)ŌĆō, and sural nerve (SN)ŌĆōsomatosensory evoked potential (SSEP) between lumbosacral (L) and control (C) groups. Means and standard deviations were shown. *p<0.05.
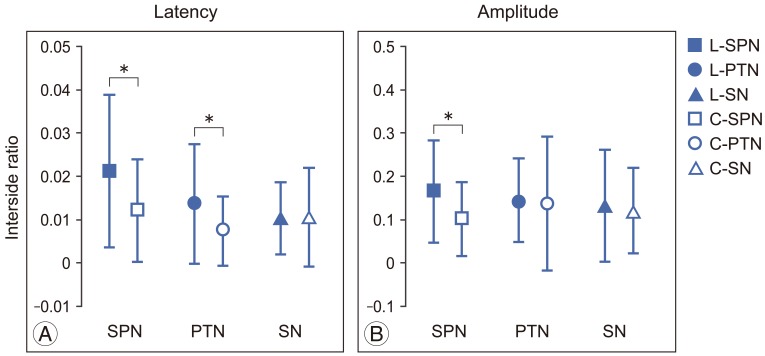
Fig.┬Ā2
Repeated measures analysis of variance analysis of the interside asymmetry ratios of superficial peroneal nerve (SPN)ŌĆō, posterior tibial nerve (PTN)ŌĆō, and sural nerve (SN)ŌĆōsomatosensory evoked potential (SSEP) P37 latencies in lumbosacral (A) and control groups (B), and P37-N45 amplitudes in lumbosacral (C) and control groups (D). Means and standard deviations were shown. *p<0.05.