Assessment of Paraspinal Muscle Atrophy Percentage after Minimally Invasive Transforaminal Lumbar Interbody Fusion and Unilateral Instrumentation Using a Novel Contralateral Intact Muscle-Controlled Model
Article information
Abstract
Study Design
Retrospective comparative clinical study.
Purpose
This study aimed to assess paraspinal muscle atrophy in patients who underwent minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) and unilateral pedicle screw fixation using a novel contralateral intact muscle-controlled model.
Overview of Literature
The increased incidence of paravertebral lumbar muscle injuries after open techniques has raised the importance of implementing minimally invasive spine surgical techniques using tubular retractors and minimally invasive screw placement. The functional cross-sectional area (FCSA) represents the lean muscle mass; furthermore, FCSA is a useful marker of the contractile ability of a muscle following a spine surgery. However, the benefits of unilateral fixation and MI-TLIF on paraspinal muscles have not been defined.
Methods
We performed a retrospective imagenological review on eleven patients who underwent unilateral MI-TLIF and unilateral transpedicular screw lumbar placement. FCSAs of the multifidus and erector spinae were measured 1 year after surgery at adjacent levels and were compared to the contralateral intact muscles. Measurement differences between the surgical and nonsurgical sites were compared. The interobserver reliability was calculated using an intraclass correlation coefficient.
Results
The mean FCSA at the surgical site was 20.97±5.07 cm2 at the superior level and 8.89±2.87 cm2 at the inferior level. The mean FCSA at the contralateral nonsurgical site was 20.15±5.95 cm2 at the superior level and 9.20±2.66 cm2 at the inferior level was. The superior and inferior FCSA measurements showed no significant difference between the surgical and nonsurgical sites (p=0.5, p=0.922, respectively).
Conclusions
Using a mini-open tubular approach through the sulcus between the longissimus and iliocostalis, MI-TLIF and unilateral pedicle screw instrumentation produced minimal paraspinal muscle damage at the superior and inferior adjacent levels.
Introduction
The multifidus and erector spinae (longissimus and iliocostalis) are essential for carrying the physiologic loads imposed on the spine; furthermore, they provide stability and functional movement [1]. The increased incidence of paravertebral lumbar muscle injuries after conventional open techniques have raised the importance of implementing minimally invasive spine surgical techniques using tubular retractors and minimally invasive screw placement [2345].
The muscle cross-sectional area (CSA) can be used to measure muscle atrophy and fatty infiltration using either computed tomography (CT) or magnetic resonance imaging (MRI) [67891011121314151617181920]. Even without a decrease in the overall CSA within the muscle fascia borders [21], atrophy can occur, indicating the replacement of muscle with fat and fibrous tissue. The functional cross-sectional area (FCSA) represents the lean muscle mass and is a useful marker of the contractile ability of a muscle [17].
This study aimed to assess paraspinal muscle atrophy using FCSA in patients who underwent a minimally invasive transforaminal lumbar interbody fusion (MI-TLIF) and unilateral pedicle screw instrumentation using a novel contralateral intact muscle-controlled model.
Materials and Methods
We performed a retrospective comparative imagenological review on eleven patients who underwent a unilateral MI-TLIF and unilateral transpedicular screw lumbar placement from January 2010 to December 2013 at the ABC Medical Center, Mexico City, Mexico.
1. Surgical technique
For the treatment of degenerative disc disease and spondylolisthesis of the lumbar spine, all patients were voluntarily treated for 1 or 2 levels using MI-TLIF. All procedures were performed under general anesthesia with patients in the supine positon on a radiolucent operating table using a two-dimensional C-arm fluoroscope, electromyography, somatosensory evoked potentials, and motor evoked potentials. Following intubation, the patient was placed in the prone position; the lumbar spine was prepared and draped in a sterile fashion. A 2-cm incision was placed 1.5 cm lateral to the ipsilateral pedicle marked by anterior-posterior fluoroscopy. A monopolar dissection was made through the subcutaneous tissue. The thoracolumbar and muscle erector spinae fasciae were opened; the plane between the iliocostalis and longissimus muscles was recognized. A 16–18-mm tubular retractor (METRx System; Medtronic, Dublin, Ireland) was inserted in between the muscles by dilating over a precisely placed guide tube over the facet joint. The facet complex was drilled out using a straight high-speed match head cutting drill. Discectomy and MI-TLIF were performed using Peek cages (JULIET OL; Spineart, Geneva, Switzerland) filled with a demineralized bone matrix. Following decompression and interbody fusion, using true AP and lateral views, fluoroscopic-guided pedicle screws were placed. The rod was secured through the same incision. The fascia and subcutaneous tissue were closed using Vycril 2-0, and the skin was closed using nylon 3-0.
2. Functional cross-sectional area protocol
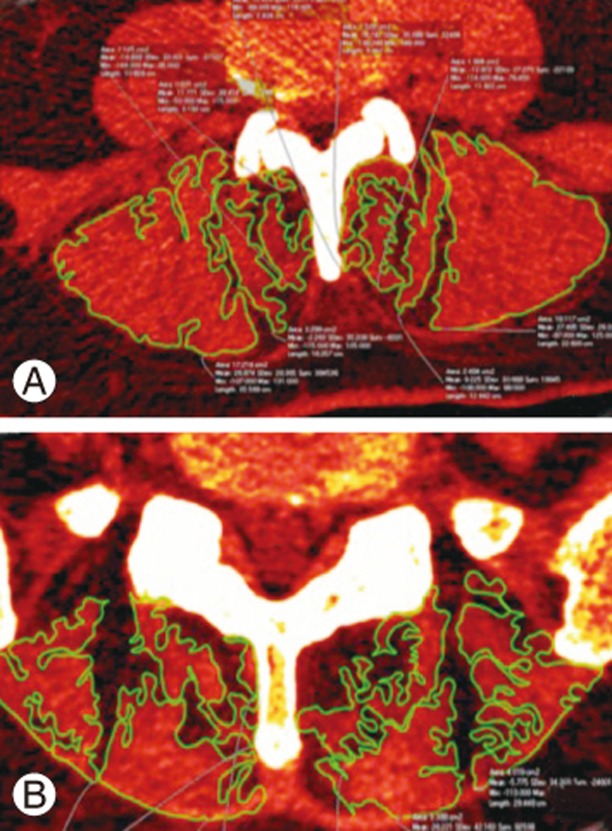
A lumbar CT (Brilliance 64 scanner; Phillips Healthcare, Andover, MA, USA) was performed 1 year after surgery. Using 5-mm thick slices, images were obtained with patients placed in the supine position. Images were stored in a DICOM (digital imaging and communications in medicine) format and analyzed on a personal computer using OsiriX ver. 5.6 (32 bit; Pixmeo SARL, Bernex, Switzerland) with the following parameters: WLWW, CT abdomen; VR muscle-bone; and opacity, logarithmic inversion. FCSA was measured in selected axial images at the superior and inferior adjacent levels to the lumbar instrumentation. Avoiding nearby fat, bony structures, and other soft tissues, FCSA was drawn with an electronic pencil (Bamboo ver. 5.2.5; Wacom Co. Ltd., Kazo, Saitama, Japan) by two independent observers blinded to the surgical method. The sum of the multifidus and erector spinae FCSAs was calculated as one muscle mass due to difficulties in discriminating between the back musculature. FCSA was bilaterally measured 1 year after surgery (Fig. 1).
3. Statistical analysis
Statistical analyses were performed using IBM SPSS Statistics Software ver. 20.0 (IBM Corp., Armonk, NY, USA). Descriptive statistics were used for clinical and demographic data. FCSA was compared 1 year after surgery for the surgical and nonsurgical sites using the Mann–Whitney U-test. The interobserver reliability was calculated using an intraclass correlation coefficient (ICC). A p-value of <0.05 was considered statistically significant.
Results
Clinical data and FCSA were evaluated in eleven patients 1 year after surgery. The mean age was 62.91±15.86 years (range, 34 to 82 years; eight males and three females). The mean surgical time was 180 minutes (range, 120 to 300 minutes); the mean bleeding volume was 40 mL (range, 20 to 200 mL).
ICC was 0.981 (range, 0.865 to 0.99), indicating excellent interobserver reliability. Therefore, the mean FCSA from the two observer's measurements was used in the statistical analysis.
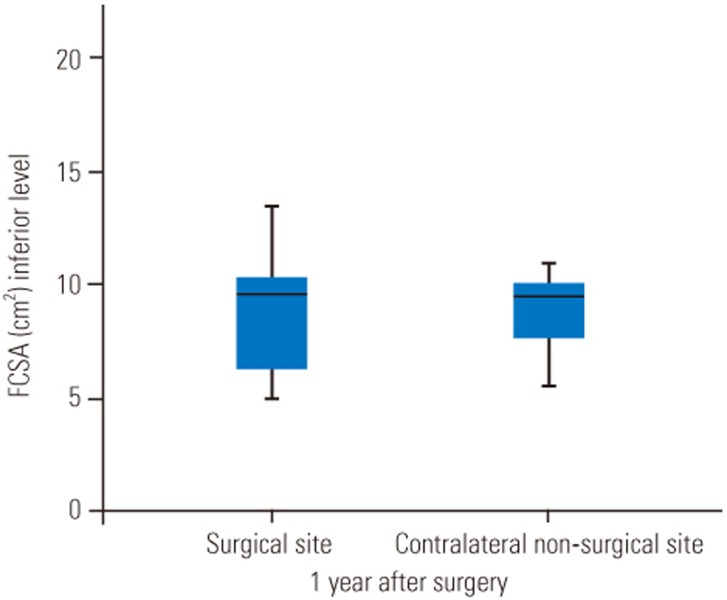
FCSA was measured at the surgical site and the contralateral side. The mean FCSA was 20.97±5.07 cm2 in the surgical site at the superior level and 8.89±2.87 cm2 at the inferior level. FCSA was 20.15±5.95 cm2 in the contralateral nonsurgical site at the superior level and 9.20±2.66 cm2 at the inferior level (Figs. 2, 3). There was no significant change between the surgical and nonsurgical sites in both superior and inferior FCSA measurements (superior level: Z=−0.624, p=0.5; inferior level: Z=−0.098, p=0.922). When using the control site as a reference, there was a +4.06% increase in surgical site muscles at the superior level and a −3.3% reduction at the inferior level.
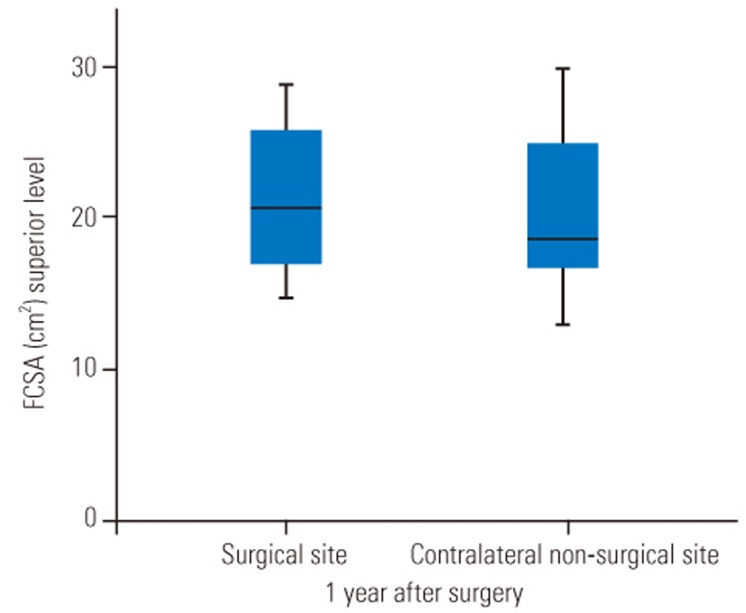
Boxplot showing FCSA after minimally invasive transforaminal lumbar interbody fusion and unilateral screw pedicle fixation at the superior adjacent level. There was a mean percentage increase of 4.06% at the surgical site (p=0.5). FCSA, functional cross-sectional area.
Discussion
It is important to assess muscle atrophy following spine surgery because there are reports on its correlation with chronic low back pain [6212223]. Although conventional open TLIF and pedicle screw fixation are beneficial, these techniques require extensive soft tissue and muscle dissection, which is associated with increased blood loss, higher infection rate, and prolonged hospital stay. Using MI-TLIF with a unilateral fixation reduces paraspinal muscles manipulation during surgery, decreasing paraspinal muscles damage, and theoretically, chronic low back pain risk.
Hu et al. [24] demonstrated that the reliability between CT and MRI to assess FCSA measurements is not significantly different and that they are acceptable methods for evaluating paraspinal muscle atrophy. Our study is a unique model that compares the surgical site with the contralateral nonsurgical site in the same patient 1 year after surgery using quantitative CT FCSA. Muscles were assessed proximal and distal to the arthrodesis; the operated level was not measured because it gave several artifacts. In patients with an abundant fatty infiltration, the muscles borders were irregular and difficult to trace; however, measurements were taken by two blinded observers with a high ICC (0.981), demonstrating that the use of previously described OsiriX parameters was adequate to identify muscle boundaries. However, differentiation between all intrinsic paraspinal muscles was difficult, particularly at the distal level (S1). Therefore, to address this problem, we assessed FCSA using the sum of all intrinsic paraspinal muscles. This measurement model wherein the contralateral side is the patient's control, overcomes age and sex differences regarding muscle volume and fat content (Fig. 1).
Using unilateral fixation with a mini-open approach prevents contralateral muscle damage, decreases surgical time and blood loss, and provides fusion. Our mini-open approach required one incision for MI-TLIF and fixation. The plane between the iliocostalis and longissimus muscles was recognized, preventing muscle damage during TLIF. The screws were placed and the rod secured through the same plane and incision. Even though contralateral fixation can be done using a mini-open or percutaneous technique, there is still an 84% and 20% risk, respectively, for a posterior rami medial branch nerve transection after pedicle screw insertion, putting the contralateral multifidus innervation at risk [25]. Cadaveric specimens showed an increased anatomic risk for medial branch nerve injury using a mini-open approach at the adjacent cranial level [25]; therefore, a unilateral fixation eliminates the risk for contralateral medial branch nerve injury, preserving the integrity and innervation of the contralateral multifidus. There were no significant differences in the fusion rate or complications between the unilateral or bilateral pedicle screw fixation [2627]; however, it is important to consider bilateral fixation in cases with preoperative instability, osteoporosis, high grade, and isthmic spondylolisthesis.
Previous studies have demonstrated a decrease in the adjacent FCSA ranging from −2% to −38% using an open approach compared with +9.9% to −12.2% using minimally invasive techniques [8914161928293031323334] (Table 1). In our study, the functional integrity assessed by FCSA showed no significant difference proximal and distal to the arthrodesis 1 year after surgery (p=0.5, p=0.922, respectively) (Figs. 2, 3).

Muscle damage assessed using clinical imaging related to open and minimally invasive spine approaches
A minimally invasive surgery aims for a simple minimal access through small incisions and to minimize substantial trauma to ligaments, muscles, and vertebrae while attaining a clinically positive effect. Using minimally invasive spine surgery (MISS) TLIF and unilateral fixation prevents contralateral damage, minimizes ipsilateral muscle atrophy, and provides good therapeutic results. The main limitations of this study are as follows: First, it was a retrospective study; direct visualization of the paraspinal muscles was not assessed at the level of fusion. Second, the results were not compared with an open technique. However, the study aimed to demonstrate minimal paraspinal muscle contractile damage using MISS TLIF and unilateral instrumentation.
Conclusions
MI-TLIF and unilateral pedicle screw instrumentation using a mini-open approach caused minimal paraspinal muscle damage at the superior and inferior adjacent levels. Cross-sectional CT imaging may contribute to the functional muscle measurement after spine surgery. Further studies are required to confirm whether muscle injury is directly related to long-term clinical outcome.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
Contents of this work will be presented as an oral presentation in the SMISS Global Forum '15, Las Vegas, Nevada, USA.