Introduction
The International Association for the Study of Pain defines pain as an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or describes it in terms of such damage. This groups committee on taxonomy goes on to state, 'If a patient regards their experience as pain and if they report it in the same way as pain caused by tissue damage, it should be accepted as pain'. Therefore, not all pain is the result of a nociceptive stimulus received and transmitted by a sensory receptor of a peripheral nerve [1,2].
The general approach to patients complaining of pain is that it is real. Pain has both physical and psychological components that may predominate and alter the personality of the individual. This change in personality usually returns to the pre-pain state when the physical cause of the discomfort has been addressed. In addition, pain always has a subjective component that is perceived by patients in relation to previous experiences with pain, usually from their early years.
This review examines the mechanisms of the pain, discusses the definitions of the terms related to pain, and evaluates the mechanisms of the analgesic effect of pain medications.
The Mechanism of Pain
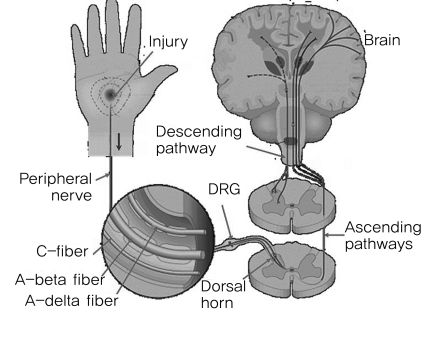
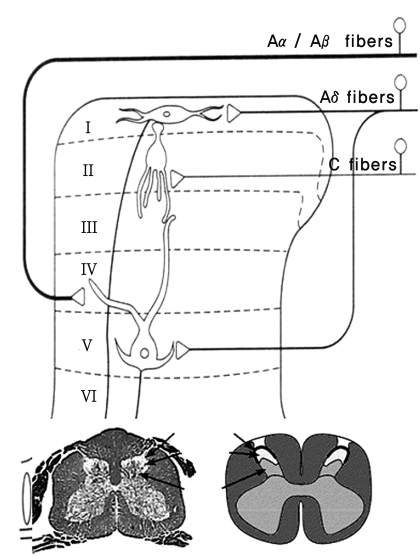
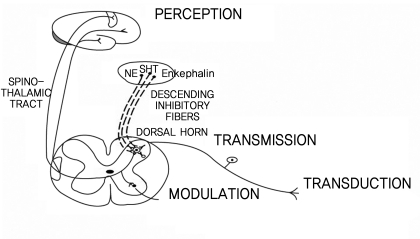
Pain is perceived and operated by the cerebral cortex through several processes; transduction, transmission, modulation and perception. The afferent fibers that convey nociception are the group A-delta and C fibers (Fig. 1). The primary afferent fibers from the peripheral nociceptors synapse with secondary order neurons at the dorsal horn in the spinal cord, conveying the pain sense [3]. Thin A-delta and C fibers synapse at laminas I and II, whereas thick A fibers synapse at lamina IV. The fibers then conduct the impulse to the brain (Fig. 2). A-beta fibers are related to chronic pain, particularly neuropathic pain.
1. Acute pain
Peripheral nociceptive stimuli eventually releases glutamate, which combines with the alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic-acid (AMPA) receptor and triggers the signal to the central nervous system. There are 4 pain inhibition mechanisms that may counteract this stimulus: the descending inhibitory pathway, hypophysis-pituitary-adrenal axis activity, glucocorticoid mediated pain inhibition by a cytokine block, and the analgesic function of the limbic system between S1. In acute pain, all 4 pain inhibition processes are working normally [4].
2. Chronic pain
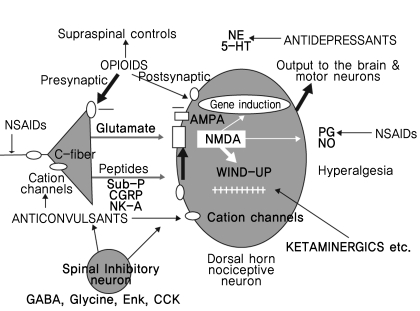
Chronic pain is mediated primarily by the N-methyl-D-aspartate (NMDA) receptor. Glutamate release stimulated by the acute pain signal cannot activate the NMDA receptor due to a magnesium ion block. Repetitive, continuous pain signals and excessive glutamate release might activate the NMDA receptor. The cascade of the intracellular calcium ion influx, protein kinase C activation, nitric oxide production and substance P release eventually occurs. The substance P released induces the expression of the c-fos gene, which promotes neural remodeling and central hypersensitization. This mechanism is responsible for converting a painful experience into a disease. In the case of chronic pain, the 4 pain inhibition mechanisms are non-functional.
Terms Related to Pain
1. Nociceptor
A nociceptor is defined as follows: "A receptor preferentially sensitive to a noxious stimulus or to a stimulus which would become noxious if prolonged." All nociceptors are specialized primary sensory neurons, whose cell bodies are located in the dorsal root or trigeminal ganglia. The primary sensory endings are normally in the periphery, and they transmit their information into the central nervous system (CNS) via central processes, such as the dorsal root ganglia or trigeminal ganglia. Nociceptors convert the mechanical, thermal, and chemical signals into action potentials that synapse in the dorsal horn (or spinal nucleus of the trigeminal nerve) onto second-order pain pathway neurons. The pain pathways eventually reach the cortex and are interpreted by the CNS as pain. In addition, the viscera and many other tissues including the spinal tissues contain "silent nociceptors" that are not normally activated by noxious stimuli. These receptors can be altered dramatically by inflammation or chemical insults, resulting in a concomitant increase in their firing rate. Therefore, these "silent nociceptors" can contribute to the development of secondary hyperalgesia or central sensitization.
2. Allodynia
Allodynia is defined as "Pain due to a stimulus which does not normally provoke pain." This term is used to describe conditions, in which a stimulus that is normally not painful is now considered painful because of some alteration in the CNS. Most nociceptive afferent fibers entering the dorsal horn undergo a high degree of divergence before synapsing on their second-order pathways. The majority of these synapse into interneurons that influence the overall neural tone of the spinal cord associated with the spinal reflexes, postural control, and the overall responsiveness of the system. In general, the responsiveness of these interneurons are normally suppressed by the descending supraspinal pathways. After an injury, the increased afferent activity associated with the nociceptive input may increase the overall output of the interneurons dramatically. Because of the large number of interneurons present in the dorsal horn and their subsequent activation by the increased nociceptive input, the afferents of the nonnociceptive fibers may subsequently activate the nociceptive interneurons, thus triggering a painful response to a normally non-painful stimulus.
3. Analgesia
Analgesia is defined as an "Absence of pain in response to stimulation which would normally be painful." Because pain is a perception controlled by the cerebral cortex, it can be modulated in many ways to alter the incoming nociceptive input and prevent the perception of a noxious stimulus. Every synapse of the nociceptive input is a potential site for modulation, and the CNS has a remarkable set of local (spinal cord) interneurons and descending modulatory pathways designed exclusively to interrupt the conveyance of nociceptive input. The perception of pain will be reduced or eliminated if the nociceptive signal can be attenuated or inhibited before reaching the cortex. In addition, exogenous agents, such as aspirin or acetaminophen, can alter the biochemical pathways responsible for the production of inflammatory agents, thereby reducing their effect on the nociceptors and thus their input into the CNS. Other agents, such as morphine, can mimic the effects of the inhibitory neurotransmitters and reduce the nociceptive input into the CNS dramatically.
4. Central pain
Central pain is defined as "Pain initiated or caused by a primary lesion or dysfunction in the central nervous system." Any disease process, trauma or space-occupying lesion affecting the CNS can alter the normal functions of the tracts, nuclei, and cell bodies of the nervous system, including the somatosensory system. CNS has no sensory afferents of its own, including nociceptors. Therefore, it cannot normally detect the presence of these abnormalities. However, changes in the normal functions indicate the presence of these pathologies. Both positive and negative signs can exist after the lesions or dysfunctions occur within the CNS. Positive signs (paresthesias) are sensations that normally are not present, but can be experienced after a lesion, and include pain, dysesthesia or tingling sensations. Negative signs are those that normally are present but are no longer perceptible, such as loss of position sense, touch or pain.
5. Hyperalgesia or sensitization
Hyperalgesia or sensitization is defined as "An increased response to a stimulus which is normally painful." Hyperalgesia differs from allodynia in that hyperalgesia is an increase in the sensitivity to pain due to a decrease in the nociceptor's threshold for pain by either peripheral or central mechanisms, whereas allodynia is a perception of pain from a non-painful stimulus. Hyperalgesia can be classified as either primary (peripheral) or secondary (central).
Primary hyperalgesia
This occurs at the site of an injury when the nociceptive fiber is sensitized or stimulated directly by chemical agents released by the damaged tissue and nearby cells or the neuron itself. Substances, such as bradykinin, histamine, prostaglandins, leukotrienes, acetylcholine, serotonin and substance P all affect the nociceptors directly by lowering their threshold for activation. After tissue damage, a nociceptor responds to the same stimulus given before the injury occurred with a greater number of action potentials. In addition, some of the substances just listed can activate the nociceptors directly, resulting in a further increase in nociceptive input into the CNS. Potassium, serotonin, bradykinin and histamine all have been identified as activators of the peripheral nociceptors. Activated nociceptors also can release chemical agents (e.g., substance P and CGRP) at the peripheral terminal that can either sensitize or activate other nearby nociceptors. These chemical agents are synthesized in the cell body (in the dorsal root ganglion) and are transported down to the peripheral terminal.
Secondary hyperalgesia
Secondary hyperalgesia occurs during severe or persistent injury. In these situations, C fibers fire repeatedly and increase the firing of neurons in the dorsal horn. This phenomenon is called "wind-up", and is dependent on the release of excitatory neurotransmitters (glutamate) from the C fibers, resulting in long-term changes in the sensitivity of the dorsal horn neurons, known as "central sensitization".
6. Hypoalgesia
Hypoalgesia is defined as "Diminished pain in response to a normally painful stimulus". Hypoalgesia can be achieved by several mechanisms, all of which involve changes in the central synapses. These alterations in central synapses also help determine the overall level of tolerance for pain, or more specifically, the threshold for the perception of pain.
7. Neuralgia
Neuralgia is referred to as "Pain in the distribution of a nerve or nerves." Pain associated with neuralgia is often described as either lancinating or paroxysmal in quality. The pain can occur spontaneously or be triggered in response to minimal stimuli that would not normally elicit pain (allodynia). The most common example is trigeminal neuralgia, or tic douloureux, which consists of excruciating, paroxysmal, electric shock-like facial pain in the distribution of one branch of the trigeminal nerve (most often the maxillary division). Pain is often evoked by a tactile stimulus in a particular location, known as the trigger zone.
8. Neurogenic pain
Neurogenic pain is defined as "Pain initiated or caused by a primary lesion, dysfunction, or transitory perturbation in the peripheral or central nervous system." Neurogenic pain normally involves the sensitization of nociceptors, dorsal root ganglia and dorsal horn neurons. This includes both central sensitization and the wind-up phenomenon. Normally, polymodal nociceptors have little or no spontaneous activity. However, after an injury they become sensitized, resulting in increased spontaneous activity, decreased threshold, increased sensitivity to heat or cold, sensitivity to sympathetic stimulation, and an antidromic release of neuropeptides. The clinical characteristics of neurogenic pain are spontaneous pain (burning, aching, or shocklike), hyperalgesia and allodynia. Neurogenic pain includes deafferentation pain, sympathetically maintained pain and neuropathic pain (Fig. 3).
Deafferentation pain
This type of pain may be a complication of an injury anywhere along the course of the somatosensory pathway. For example, phantom limb pain is associated with the peripheral nerves and spinal cord dysfunction after amputation, whereas multiple sclerosis can cause dysesthesia and pain resulting from demyelination in the spinal cord or brain stem. In addition, thalamic syndromes can lead to a severe burning sensation (dysesthesia) after a localized lesion within the thalamus.
Sympathetically maintained pain
This condition is most often characterized by the simultaneous occurrence of pain, local autonomic dysregulation (e.g., edema, vasomotor disturbances, abnormal sweating) and trophic changes in the skin, soft tissues and bone. These changes are most often associated with lesions of the peripheral nerves or nerve roots. Normally most nociceptive afferents show considerable divergence as they enter the dorsal horn, and many nociceptive afferents activate directly or indirectly multiple interneurons, reflex pathways, second-order neurons and sympathetic neurons. The activation of these sympathetic neurons in the intermediolateral cell column causes changes in blood flow (vasoconstriction or vasodilation, as well as swelling, edema and possibly trophic changes) that assist in healing. However, this also contributes to sensitization and direct activation of the nociceptors at and near the site of the injury (primary hyperalgesia), resulting in increased sensitivity to pain. When these normal physiological responses continue past the time of healing, they can become pathological in nature and contribute to sympathetically maintained pain. Two types have been recognized: reflex sympathetic dystrophy or complex regional pain syndrome (CRPS) I, and causalgia or CRPS II. CRPS I develops after an initiating noxious event, whereas CRPS II develops after a nerve injury.
Neuropathic pain
Neuropathic pain is defined as" Pain initiated or caused by a primary lesion or dysfunction in the nervous system." It is a type of neurogenic pain that is normally associated with painful nerve compression (e.g., Intervertebral disc compression of a dorsal root or dorsal root ganglion) or after the formation of posttraumatic neuromas subsequent to a nerve lesion. This results in abnormal firing of the nerves in the areas of demyelination, selective loss of large fiber-mediated segmental inhibition, and an increased activity of the small nociceptive fibers. The increased activity of the nociceptive fibers is believed to be mediated by local changes in extracellular ionic concentrations (increased Na+ and decreased K+ ions), which result in depolarization of the unmyelinated and demyelinated fibers [5].
9. Nociceptive pain
Nociceptive pain is defined as "Pain induced by direct stimulation of nociceptive afferents." Portenoy and Kanner [6] defined nociceptive pain as "pain that is believed to be commensurate with the presumed degree of ongoing activation of peripheral nociceptors." Nociceptive pain is related directly to the activation of normal pain mechanisms in response to tissue injury. Therefore, any injury or condition that causes an increase in the normal activity of the nociceptive afferents may cause nociceptive pain if that increase in activity is recognized by the cortex. Indeed, most nociceptive pain is often more severe than the perceived intensity of the stimulus due to the extensive network of inhibitory connections that exist to alter the threshold for the perception of pain. The severity of an injury is not always related directly to the amount of pain perceived by the subject. Some overt injuries can produce an obvious painful condition. However, there are many stimuli (e.g., changes in the local extracellular ion concentrations) that can activate the nociceptors without obvious trauma or injury to the site of the painful condition. Acute pain is most often attributable to some trauma or altered condition that activates the nociceptors directly [7]. However, chronic pain is more difficult to define and treat because the initial cause of the pain (activation of nociceptors) can be altered dramatically by central mechanisms. Stimulation of the nociceptors resulting from an initial injury may be dissociated from the ongoing perception of pain long after the injury has healed due to changes in the second-order fibers, interneurons and descending pathways. Therefore, chronic pain may no longer be attributable to nociceptive pain.
Many of the structures in and around the lower back are potential pain generators that can initiate nociceptive pain. These structures include the muscle, tendons, joint capsules, periosteum, perivenous tissues (nervi vasorum), visceral tissues, transverse and spinous processes, spinal ligaments, Z joints, as well as the intervertebral discs and nerves themselves (nervi nervorum).
10. Pain threshold
The pain threshold is defined as "The least experience of pain which a subject can recognize." The threshold was described traditionally as the lowest stimulus intensity at which a subject would perceive pain. This term (stimulus intensity) was excluded from the current definition because the stimulus intensity is an external measure used to elicit a response from the subject and cannot be a measure of pain. However, pain is an experience defined by the subject, and consequently, highly subjective. Therefore, the threshold is also highly subjective and related directly to the subject's current disposition. A subject who is energetic, feeling good, and in good health will most likely have a higher tolerance (higher threshold) to pain than a subject who is tired, injured, or suffering the effects of a cold, flu, or other seemingly unrelated illnesses.
Treatment
1. Gate control theory
Pain transmission cells (T-cells) are located in the dorsal horn of the spinal cord. The T-cells receive a nociceptive stimulus from both peripheral pain sensory fibers (small-diameter fiber, S-fiber) and non-nociceptive sensory nerve fibers (large-diameter fiber, L-fiber). Briefly, the gate control theory states that the L-fibers decrease the afferent sensory input to the T-cell by stimulating the inhibitory interneuron at the substantia gelatinosa (SG), whereas the S-fibers increase the afferent sensory input by inhibiting the inhibitory interneuron at the SG [6].
This concept has led to effective treatments for the relief of pain, such as transcutaneous electrical nerve stimulation (TENS) and dorsal column stimulation. TENS increases the activity of the L-fibers, and competitively inhibits pain-conducting fibers (S-fibers) by stimulating the peripheral sensory nerve at low intensity and high frequency. Dorsal column stimulation activates the inhibitory interneurons at the SG, which then decrease the activity of the T-cells [8].
2. Analgesics
Analgesics are defined as substances that "cause and absence or deadening of pain without a loss of consciousness." Figures 4 and 5 describe the action mechanisms of each drug [9,10].
Morphinergic drugs
These drugs participate in the pathway between the internal analgesic system, and macula nucleito spinal cord. The internal analgesic system exists paraaxially at the gray mater around central canal, upper medulla (raphe nuclei) and spinal cord [11].
Tricyclic antidepressants
These drugs activate the descending inhibitory pathway that normally works during acute pain. Therefore, they are effective in neuropathic pain but are contraindicated in narrow angle glaucoma, benign prostatic hypertrophy, and an acute myocardial infarction [12].
Anticonvulsants
Drugs, such as phenytoin, carbamazepine, block the neurotransmitter and ion channels.
Gabapentin
Gabapentin is an example of an anticonvulsant that acts on the spinal cord inhibitory interneurons. Caution is needed when administering Gabapentin to patients diagnosed with nephrotic syndrome.
Local anesthetics
Sensory impulses from the peripheral nociceptors are converted into pain fiber action potentials. These action potentials are governed by voltage-gated Na+ channels, which are blocked by local anesthetics. Caution should be taken when administering these to patients with cardiac conduction abnormalities, as well as left ventricle and renal insufficiency.
NMDA receptor inhibitors
Drugs, such as ketamine, dextromethorphan, reduce pain by blocking the NMDA receptor at the dorsal horn of the spinal cord.
Steroids
After a tissue injury, activated phospholipase A catalyzes the conversion of prostaglandins from arachidonic acid via the cyclooxygenase pathway. Prostaglandins are known to induce pain. A steroid blocks the conversion of arachidonic acid to prostaglandins. Recently, it was discovered that the lipooxygenase pathway may also contribute to the production of pain.
Conclusions
Pain is an early signal and a red alert to protect an individual from tissue injury. The causative factors initially activate the pain mechanisms but pain itself is caused by a variety of mechanisms. Therefore, same diseases produce various types of pain via a range of pain mechanisms, and some of the characteristics of pain are produced by a variety of causative diseases. The treatment strategy of pain should be directed to eradicating both the causative diseases and the symptoms of pain itself.