|
 |
- Search
| Asian Spine J > Volume 5(2); 2011 > Article |
Abstract
Study Design
We performed an ex vivo study to observe cell morphology and viability of human nucleus pulposus (NP) chondrocytes isolated from degenerated intervertebral discs (IVD).
Overview of Literature
Biological repair of IVDs by cell-based therapy has been shown to be feasible in clinical trials. As one of the most promising transplanting seeds, how the isolated NP chondrocytes behavior ex vivo has not been fully understood.
Methods
Human NP chondrocytes were harvested from 20 degenerated IVDs and cultured in monolayers. Histological and immunochemistry staining was used to detect cell morphology change. Cell viability was studied by analyzing cell cycle distribution and apoptotic rate in the primary and subculuted cells.
Results
The round or polygonal primary NP chondrocytes had an average adherence time of 7 days and took nearly 31 days to reach 95% confluence. The spindle-shaped P1 NP chondrocytes increased growth kinetics and took about 12 hours to adhere and 6.6 days to get 95% confluent. Immunochemistry staining of collagen II was positive in the cell cytoplasm. Nearly 90% of the confluent NP chondrocytes stayed in G1 phase while 16% underwent apoptosis. No significant difference of the collagen II expression, cell cycle distribution or the apoptosis indices were detected between the primary and subcultured NP chondrocytes.
Disorders related to low back pain (LBP) are imposing a great economic burden on health care system. In the United States, about $19.6-50 billion in annual health costs are directly or indirectly accredited to LBP [1]. Although more than 20% of all LBP originates from the degenerative change of intervertebal discs (IVDs), the exact mechanism by which the IVD degenerates remains largely unknown [2].
Healthy human IVDs contain a small cell population that occupies less than 1% of the total disc volume [3]. These cells are surrounded by an extracellular matrix (ECM) that mainly consists of collagens and proteoglycans. With aging and degeneration, a gradual process of cellular loss and degrading the ECM happens within the IVD [3,4].
In order to reverse further decrease of cell numbers and to restore the functional matrix content, biological repair of IVDs by cell-based therapy has been shown to be feasible in clinical trials [5]. As reported in the EuroDISC multi-center study, percutaneous reinjection of autologous disc cells potentially provides long-term pain relief and this maintains disc height. More efficient ways to get enough qualified seed cells and a comprehensive understanding of their biological behaviors are becoming more and more fundamental to the future clinical applications [2,5].
Throughout life the disc cells are constantly challenged by a stressful microenvironment such as hypoxia [6], a limited supply of nutrition [7], and various mechanical loadings [8]. An extensive spectrum of apoptosis and cellular senescence changes is observed in response to those specific microenvironments [9,10]. Besides that, a network of cellular function change has also been demonstrated by many in vivo studies (Fig. 1). These changes include, but are not limit to the increased inflammatory cytokines and activated matrix degrading enzymes [11-13], decreased anabolic abilities and lost chondrocyte phenotypes [14,15]. Although the alteration of cell biological behavior is supposed to trigger and/or accelerate the degeneration change within IVDs, our current information about how the isolated cells behavior ex vivo and their potential effects on their clinical or experimental use is limited [15-19]. The present work aimed to observe human nucleus pulposus (NP) chondrocytes in monolayer cultures and provide more information about their ex vivo biological behaviors.
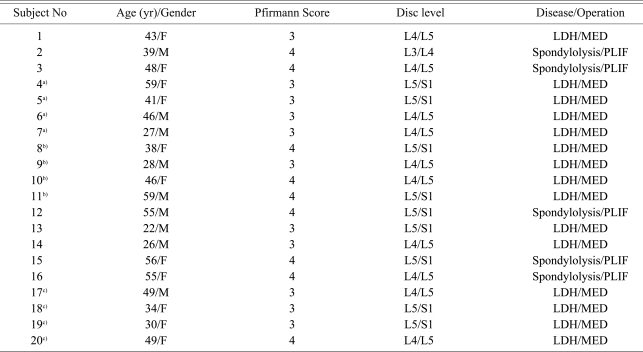
IVDs from 20 donors were collected from Orthopedic Department of Zhongda hospital. Fifteen donors underwent microendoscopic discectomy (MED) for lumber disc herniations. Posterior lumber interbody fusion (PLIF) was performed in five patients with spondylolysis (Table 1). Pfirrmann's classification was used to grade the extent of disc degeneration in each subject [20]. Disc tissues were collected under sterile conditions and they were transported in high glucose Dulbecco modified Eagle medium (HG-DMEM, Gibco-BRL, Rockvill, MD, USA) at 0Ōäā. All the biopsies were delivered to the laboratory for processing of the cell cultures within 30 minutes. This study was approved by Southeast University's ethics committee and informed written consent was obtained from all the donors.
The NP tissues were identified by their macroscopic morphology and they were carefully separated from the endplates or annulus. After being washed twice in phosphate buffered saline solution (PBS, Sunbio, Beijing, China), the selected tissues were digested at 37Ōäā by incubation for 4 hours in 0.025% human type II collagenase (Sigma-Aldrich, St. Louis, MO, USA). The released cells were filtered through a 100 ┬Ąm cell-strainer and centrifuged at 1,500 rpm for 5 minutes, and then they were suspended in HG-DMEM and counted. HG-DMEM containing 10% fetal bovine serum (Sijiqing Biological Engineering Materials Co., Hangzhou, China), 100 ┬Ąg/ml penicillin and 100 ┬Ąg/ml streptomycin was used as the cell culture medium. All the cells were seeded at 5,000 cells/cm2 and cultured at 37Ōäā under conditions of 95% humidity and 5% CO2. The culturing media was replaced twice a week, with the exception that the primary cells were allowed more time (6.9 ┬▒ 1.1 days) to adhere before the first change of the culture media. The confluent cells were dissociated by 5% trypsin (Sigma-Aldrich) that contains 1% ethylenediaminetetraacetic acid (EDTA), and they were split 1 : 2 for subculturing.
For the morphology observation, 6-well culture plates (Corning Co., Lowell, MA, USA) with an additional coverglass in each well were used. The primary or P1 NP chondrocytes that adhered on the coverglass were experimented with, respectively, for (1) observation of the morphological change under an inverted phase contrast microscope (Zeiss, Jena, Germany), (2) hematoxylin and eosin (HE) staining: briefly, the coverglasses were washed with PBS then they were fixed in 4% paraformaldehyde for 30 minutes, followed by consecutive staining in hematoxylin and eosin, (3) toluidine blue staining: the coverglasses that were washed and fixed as in the HE staining were immersed for 2 hours in 1% toluidine blue solution (KeyGen Biotech Co., Nanjing, China), and then they were rinsed in 95% ethanol, (4) immunohistochemistry staining of collagen II: the endogenous peroxidase was blocked by 3% H2O2 in methanol, and then the coverglasses were incubated for 30 minutes with anti-human type II collagen antibody (Abcam, Cambridge, MA, USA) at a 1 : 50 dilution. The secondary antibody linked with avidin-biotin-peroxidase (Abcam) and diaminobenzadine substrate solutions were used to visualize the immunoreactivity, followed by counterstaining in hematoxylin. Negative control was processed without anti-human type collagen antibody. Based on the optical density of the staining, the collagen II expressions were quantified by a LeicaQ550IW image analysis system using LeicaQwin Pro ver. 2.2 software (Leica Microsustems Imaging Solutions Ltd., Cambridge, UK).
The NP chondrocytes were cultured in 75 cm2 flask (Corning Co.) before used for (1) growth kinetics observation: the average time to adhere and to reach 95% confluent was compared between the primary and P1 NP chondrocytes. (2) Analysis of cell cycle distribution: a KGA511 PI kit (KeyGen Biotech Co.) and flow cytometry were used for the cell cycle analysis. Briefly, the cells were detached by 5% trypsin with 1% EDTA and they were fixed with 70% ice-cold ethanol, then they were digested with 100 ┬Ąl RNase A at 37Ōäā for 30 minutes and stained with 100 ┬Ąl propidium iodide (PI) for 30 minutes in dark. The filtered samples were then analyzed for cell cycle content on a FACScan cell sorter using Cell Quest software (Becton Dickinson, Mountain View, CA, USA), and the percentages of cells in the G1, S, and G2/M phase were determined by ModFit LT software (Verity Software House, Topsham, ME, USA). (3) Detection of apoptotic rate: cellular apoptosis was identified by the KGA107 Annexin-V/PI kit (KeyGen Biotech Co.) and quantified by flow cytometric analysis. According to the manufacturer's instructions, the cells were detached by 5% trypsin without EDTA and next centrifuged at 2,000 rpm for 5 minutes, and then the cell pellet was suspended in 500 ┬Ąl binding buffer and reacted with 5 ┬Ąl annexin V-FITC and 5 ┬Ąl PI at room temperature for 10 minutes in dark, followed by counting of 10,000 cells using a FACScan cell sorter within 1 hour. The apoptotic cells, including those staining positive for annexin V-FITC and negative for PI and those that were double positive, were counted and represented as a percentage of the total cell count.
All the data is presented as mean values ┬▒ standard deviation of independent experiments. Differences were considered significant when the p < 0.05, based on paired Student's t-test for comparison of two samples. All the analyses were performed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
All of the 20 NP tissues were digested by 0.025% type II collagenase and NP chondrocytes were released. The primary NP chondrocytes in 4 NP tissues, all harvested from the herniated L4/5 discs by MED, failed to adhere before the first time of replacing the culture media. The isolated cells from the other 16 NP tissues were successfully cultured and subcultured. Neither over-digestion nor germ contamination was evident during the processing of the cell culture.
The freshly isolated NP chondrocytes were rounded and mixed up with a certain number of red blood cells. After adherence, NP chondrocytes became polygonal with short processes or changed to a spindle shape (Fig. 2). The adhered NP chondrocytes were then purified by washing away the red blood cells during changing culture media. To visualize the cell morphology change in the monolayer cultures, the cytoplasm of NP chondrocytes was stained red by HE staining, blue by toluidine blue staining and brown by type II collagen immunochemistry staining respectively (Fig. 3). More NP chondrocytes were observed to demonstrate a spindle shape morphology as the cells proliferated and were subcultured. No change in type II collagen expressions was seen between the primary (151.64 ┬▒ 15.07, n = 16) and P1 (151.24 ┬▒ 14.47, n = 16) NP chondrocytes (p > 0.05).
When seeded at the density of 5,000 cells/cm2 and cultured under unified conditions, the primary NP chondrocytes adhered much slower (6.9 ┬▒ 1.1 days, n = 16) than did the subcultured (0.5 ┬▒ 0.1 days, n = 16) NP chondrocytes (p < 0.05). The P1 NP chondrocytes also demonstrated increased growth kinetics by more quickly reaching (6.6 ┬▒ 2.3 days, n = 16) to 95% confluent than that of the primary (31.3 ┬▒ 7.0 days, n = 16) NP chondrocytes (p < 0.05).
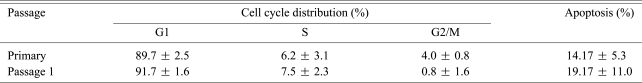
The NP chondrocytes from 8 patients were chosen to analyze cell cycle distribution and the cellular apoptosis rate in the monolayer cultures. When the NP chondrocytes reached 95% confluent, about 90% of the cells were in the G1 phase while 16% of the cells underwent apoptosis (Table 2). No significant difference was noticed between the primary and subcultured NP chondrocytes in terms of the cell cycle distribution and the apoptotic rate.
For the biological repair of IVD degeneration, NP chondrocytes have been widely used to test the feasibility of gene therapy [21], injecting growth factors [22], and the transplantation of autologous cells [5]. Although disc cells can be harvested from IVDs by several different methods [15-19], our current understanding about their ex vivo biological behavior is quite limited. In this study, we isolated human NP chondrocytes by 0.025% type II collagenase digestion and we had an 80% (16/20) rate of success. The monolayer culture system was chosen to provide the most fundamental information about their ex vivo behavior because three-dimension cultures and bioreactors have been reported to significantly influence the biological response of disc cells [18,23].
Despite the lack of specific molecular markers to identify cells of IVD origin, NP chondrocytes are generally accepted to be chondrocyte-like cells that constitutively express collagen II and proteoglycans [2]. Previous studies also noticed a characteristic dedifferentiation of NP chondrocytes in monolayer cultures [16-18]. In this present work, the chondrogenic phenotype of the freshly isolated human NP chondrocytes was evidenced by the rounded morphology and type II collagen staining. After being proliferated and subcultured in monolayers, the NP chondrocytes started to demonstrate the fibrotic phenotype by changing to a spindle shape morphology and there was a sharp increase of the growth kinetics. However, in contrast to the findings by Kluba et al. [18], no significant decrease of the type II collagen expression was found in the subcultured NP chondrocytes. This was probably due to the limited culture period and the detection methods used in our study.
Clinical trials of transplanting NP chondrocytes have highlighted that a sufficient number of qualified seed cells are prerequisite for cell based therapy [2,5]. Since NP chondrocytes quickly dedifferentiates in monolayers, accumulated proliferation or prolonged culture will eventually detract from the chondrocyte nature of the NP chondrocytes. Discectomy procedures like MED and PLIF have provided convenient access for cell collection. However, more efficient ways to culture and expand human NP chondrocytes are still not available [15-19]. Our study revealed at least two difficulties during the process of culturing human NP chondrocytes. First, the freshly isolated cells were easily contaminated by red blood cells. Although these blood cells were gradually washed away when changing the culture medium, contamination of non-disc cells potentially delay the early purification and utilization of primary NP chondrocytes. Second, the primary NP chondrocytes were difficult to adhere and they propagated slowly in a monolayer. We found that freshly isolated cells took an average of one week to adhere and one month to reach confluent. An important contributor to the slow growth kinetics was that most of the primary cells (about 90%) did not adhere before the first replacement of culture medium. Although they were given more time (6.9 ┬▒ 1.1 days) to adjust themselves to the monolayer environment, the cells from 4 NP tissues eventually failed to adhere and they had to be washed away during the change of culture medium. We once used a polylysine coated flask to culture the human NP chondrocytes, but no improvement in cell adherence was detected (data not shown).
Our understanding about the IVD pathophysiology is mainly based on the biological behaviors of disc cells [3,4,6-10]. In vivo studies have demonstrated a network of cell function changes and accumulation of apoptotic or senescent cells during IVD degeneration [9,10,13,14]. To date, it remains largely unknown whether these disc cells maintain or change their biological behaviors after being isolated from an IVD. Here we found that when the NP chondrocytes reached 95% confluent in monolayer cultures, nearly 90% of the cells stayed in G1 phase and 16% underwent apoptosis. The confluent cells were chosen for study since the time of 95% confluence, and this is the generally accepted time point for subculture or experiment use.
Some researchers have suggested that cells from more degenerative discs were poorly suited for IVD regeneration [10]. The high index of apoptosis in the cultured NP chondrocytes supported this point of view. Unlike apoptosis, which symbolizes the ending of cellular events [9], senescence is a programmed process of permanent G1-S transition arrest [10]. Senescent cells no longer proliferate, but they still maintain their metabolic activity and they stay long enough to accumulate within the IVD [24,25]. Although we did not quantify the senescent rate by senescence-associated ╬▓-gal staining [10,24], a dominant distribution in the G1 cycle was evidenced in the ex vivo degenerated human NP chondrocytes. Le Maitre et al. [24] reported that cell turnover was extremely slow in the IVD. The G1-S transition arrest of NP chondrocytes potentially contributes to their low growth kinetics. Interestingly, we observed an increase of growth kinetics in the subcultured NP chondrocytes, but we detected no significant improvement in the G1-S transition. Our data suggests that it is the adherence efficiency other than the cell cycle distribution that mostly influences cellular expansion in monolayer cultures. Further work is underway in our lab to investigate why most of the primary NP chondrocytes can not adhere and whether or not they are all senescent.
This research helps us to better understand the biological behavior of human NP chondrocytes in monolayer cultures. Change in cell morphology and growth kinetics reveal the difficulties in the ex vivo expansion of degenerated disc cells. Cell viability in terms of apoptosis or senescence should be taken into consideration when these cells are utilized for testing the feasibility of biological repair of IVDs. Future work should provide more information about the changes of behavior of disc cells after they are isolated from IVDs.
Acknowledgements
This work was supported by two grants from the national nature science foundation of China (No.81071493 and NO.31070876). The manuscript submitted does not contain information about medical device(s)/drug(s). Foundation funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
1. Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J 2008 8:8ŌĆō20. PMID: 18164449.


2. Sakai D. Future perspectives of cell-based therapy for intervertebral disc disease. Eur Spine J 2008 17(Suppl 4): 452ŌĆō458. PMID: 19005704.



3. Bibby SR, Jones DA, Lee RB, Yu J, Urban JP. The pathophysiology of the intervertebral disc. Joint Bone Spine 2001 68:537ŌĆō542. PMID: 11808995.


4. Zhao CQ, Wang LM, Jiang LS, Dai LY. The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev 2007 6:247ŌĆō261. PMID: 17870673.


5. Meisel HJ, Siodla V, Ganey T, Minkus Y, Hutton WC, Alasevic OJ. Clinical experience in cell-based therapeutics: disc chondrocyte transplantation A treatment for degenerated or damaged intervertebral disc. Biomol Eng 2007 24:5ŌĆō21. PMID: 16963315.


6. Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res 2007 22:1851ŌĆō1861. PMID: 17592963.


7. J├╝nger S, Gantenbein-Ritter B, Lezuo P, Alini M, Ferguson SJ, Ito K. Effect of limited nutrition on in situ intervertebral disc cells under simulated-physiological loading. Spine (Phila Pa 1976) 2009 34:1264ŌĆō1271. PMID: 19455001.


8. Wang DL, Jiang SD, Dai LY. Biologic response of the intervertebral disc to static and dynamic compression in vitro. Spine (Phila Pa 1976) 2007 32:2521ŌĆō2528. PMID: 17978649.


9. Zhao CQ, Jiang LS, Dai LY. Programmed cell death in intervertebral disc degeneration. Apoptosis 2006 11:2079ŌĆō2088. PMID: 17051327.


10. Roberts S, Evans EH, Kletsas D, Jaffray DC, Eisenstein SM. Senescence in human intervertebral discs. Eur Spine J 2006 15(Suppl 3): S312ŌĆōS316. PMID: 16773379.


11. Hoyland JA, Le Maitre C, Freemont AJ. Investigation of the role of IL-1 and TNF in matrix degradation in the intervertebral disc. Rheumatology (Oxford) 2008 47:809ŌĆō814. PMID: 18397957.


12. Wako M, Haro H, Ando T, et al. Novel function of TWEAK in inducing intervertebral disc degeneration. J Orthop Res 2007 25:1438ŌĆō1446. PMID: 17568422.


13. Sowa G, Vadal├Ā G, Studer R, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine (Phila Pa 1976) 2008 33:1821ŌĆō1828. PMID: 18670334.


14. Tolonen J, Gr├Čnblad M, Vanharanta H, et al. Growth factor expression in degenerated intervertebral disc tissue: an immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J 2006 15:588ŌĆō596. PMID: 15980999.


15. Cheng CC, Uchiyama Y, Hiyama A, Gajghate S, Shapiro IM, Risbud MV. PI3K/AKT regulates aggrecan gene expression by modulating Sox9 expression and activity in nucleus pulposus cells of the intervertebral disc. J Cell Physiol 2009 221:668ŌĆō676. PMID: 19711351.



16. Gruber HE, Stasky AA, Hanley EN Jr. Characterization and phenotypic stability of human disc cells in vitro. Matrix Biol 1997 16:285ŌĆō288. PMID: 9501328.


17. Gruber HE, Hanley EN Jr. Human disc cells in monolayer vs 3D culture: cell shape, division and matrix formation. BMC Musculoskelet Disord 2000 1:1PMID: 11231882.



18. Kluba T, Niemeyer T, Gaissmaier C, Gr├╝nder T. Human anulus fibrosis and nucleus pulposus cells of the intervertebral disc: effect of degeneration and culture system on cell phenotype. Spine (Phila Pa 1976) 2005 30:2743ŌĆō2748. PMID: 16371897.


19. Preradovic A, Kleinpeter G, Feichtinger H, Balaun E, Krugluger W. Quantitation of collagen I, collagen II and aggrecan mRNA and expression of the corresponding proteins in human nucleus pulposus cells in monolayer cultures. Cell Tissue Res 2005 321:459ŌĆō464. PMID: 16001263.


20. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976) 2001 26:1873ŌĆō1878. PMID: 11568697.


21. Moon SH, Nishida K, Gilbertson LG, et al. Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine (Phila Pa 1976) 2008 33:1850ŌĆō1855. PMID: 18622355.



22. Wei A, Brisby H, Chung SA, Diwan AD. Bone morphogenetic protein-7 protects human intervertebral disc cells in vitro from apoptosis. Spine J 2008 8:466ŌĆō474. PMID: 18082466.


23. Yang SH, Wu CC, Shih TT, Chen PQ, Lin FH. Three-dimensional culture of human nucleus pulposus cells in fibrin clot: comparisons on cellular proliferation and matrix synthesis with cells in alginate. Artif Organs 2008 32:70ŌĆō73. PMID: 18181806.


24. Le Maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther 2007 9:R45PMID: 17498290.



25. Kim KW, Chung HN, Ha KY, Lee JS, Kim YY. Senescence mechanisms of nucleus pulposus chondrocytes in human intervertebral discs. Spine J 2009 9:658ŌĆō666. PMID: 19540815.


Fig.┬Ā1
The current understanding of biological behavior of nucleus pulposus chondrocytes within the intervertebral disc (IVD). A healthy human IVD contains a small cell population that occupies less than 1% of the total disc volume. Throughout life, disc cells are constantly challenged by a stressful microenvironment such as hypoxia, a limited supply of nutrition and various mechanical loadings. In response to those specific microenvironments, an extensive index of apoptosis and cellular senescence is observed. Besides that, a network of cellular function change has also been demonstrated by many in vivo studies. Our understanding about IVD pathophysiology is mainly based on the biological behaviors of disc cells, which are being widely tested for use to biologically repair IVD degeneration. However, it remains largely unknown whether these disc cells maintain or change their biological behaviors after isolated from IVD. RB: Retinoblastoma, TNF-╬▒: Tumor necrosis factor-╬▒, TNFR: TNF receptor, IL-1╬▓: Interleukin-1╬▓, TWEAK: Tumor necrosis factor-like weak inducer of apoptosis, MMP: Matrix metalloproteinases, TIMP: Tissue inhibitors of metalloproteinases, IGF-1: Insulin growth factor-1, IGFR: IGF receptor; Col: Collagen.
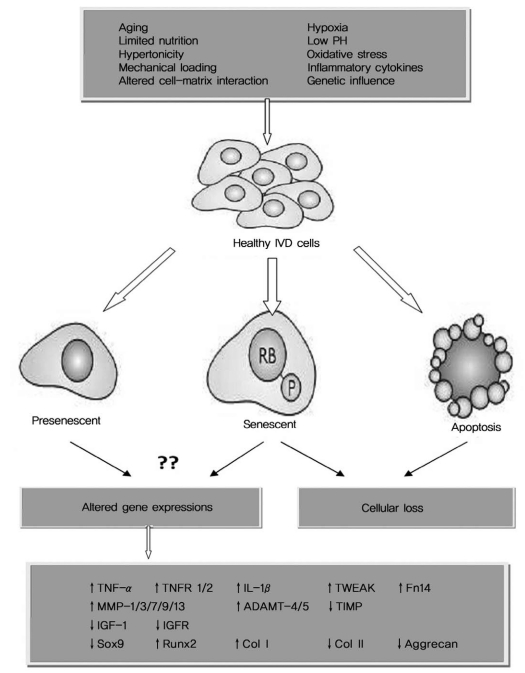
Fig.┬Ā2
Morphologic change of human nucleus pulposus (NP) chondrocytes in a monolayer culture (under an inverted phase contrast microscope). The freshly isolated NP chondrocytes (A) were rounded (black arrow) and mixed up with a certain number of red blood cells (white arrow). After adherence (B), the NP chondrocytes became polygonal with short processes (black arrow) or they changed to a spindle shape (white arrow); As the primary NP chondrocytes proliferated (C) and subcultured (D), more cells demonstrated a spindle shape (white arrows) in the monolayer cultures (├Ś250).

Fig.┬Ā3
Cytochemistry staining of the primary (A, C, E) and subcultured (B, D, F) nucleus pulposus (NP) chondrocytes. To visualize the cell morphologic changes in the monolayer cultures, the cytoplasm of the NP chondrocytes was stained red by hematoxylin and eosin staining (A, B; ├Ś200), blue by toluidine blue staining (C, D; ├Ś250) and brown by type II collagen immunochemistry staining (E, F; ├Ś200), respectively. The rounded or polygonal (black arrows) primary NP chondrocytes changed to a spindle shape (white arrows) after subculture. No significant decrease in the type II collagen expressions was detected between the primary and passage 1 human NP chondrocytes.