Comparison of Clinical and Radiological Results of Posterolateral Fusion and Posterior Lumbar Interbody Fusion in the Treatment of L4 Degenerative Lumbar Spondylolisthesis
Article information
Abstract
Study Design
Multicenter analysis of two groups of patients surgically treated for degenerative L4 unstable spondylolisthesis.
Purpose
To compare the clinical and radiographic outcomes of posterolateral fusion (PLF) and posterior lumbar interbody fusion (PLIF) for degenerative L4 unstable spondylolisthesis.
Overview of Literature
Surgery for lumbar degenerative spondylolisthesis is widely performed. However, few reports have compared the outcome of PLF to that of PLIF for degenerative L4 unstable spondylolisthesis.
Methods
Patients with L4 unstable spondylolisthesis with Meyerding grade II or more, slip of >10° or >4 mm upon maximum flexion and extension bending, and posterior opening of >5 degree upon flexion bending were studied. Patients were treated from January 2008 to January 2010. Patients who underwent PLF (n=12) and PLIF (n=19) were followed-up for >2 years. Radiographic findings and clinical outcomes evaluated by the Japanese Orthopaedic Association (JOA) score were compared between the two groups. Radiographic evaluation included slip angle, translation, slip angle and translation during maximum flexion and extension bending, intervertebral disc height, lumbar lordotic angle, and fusion rate.
Results
JOA scores of the PLF group before surgery and at final follow-up were 12.3±4.8 and 24.1±3.7, respectively; those of the PLIF group were 14.7±4.8 and 24.2±7.8, respectively, with no significant difference between the two groups. Correction of slip estimated from postoperative slip angle, translation, and maintenance of intervertebral disc height in the PLIF group was significantly (p<0.05) better than those in the PLF group. However, there was no significant difference in lumbar lordotic angle, slip angle and translation angle upon maximum flexion, or extension bending. Fusion rates of the PLIF and PLF groups had no significant difference.
Conclusions
The L4–L5 level posterior instrumented fusion for unstable spondylolisthesis using both PLF and PLIF could ameliorate clinical symptoms when local stability is achieved.
Introduction
Surgical treatment of degenerative lumbar spondylolisthesis is controversial. Such procedures include decompression alone or with fusion (in-situ fusion or fusion with correction). Simple posterior decompression in patients without lumbar instability is reported to achieve good short-term results. However, symptoms can recur within a few years [12]. Thus, current consensus is that fusion is necessary for degenerative lumbar spondylolisthesis.
Development of pedicle screws has improved clinical results [34]. Posterior lumbar interbody fusion (PLIF) that employs interbody fusion and posterior lateral fusion (PLF) that does not employ interbody fusion are widely performed. However, which one is an optimal surgical approach remains controversial.
Anterior column support by PLIF is expected to achieve better clinical results than PLF as it can achieve lumbar lordosis recovery, slip correction, and indirect foraminal decompression [5]. In addition, bone graft after debridement of degenerated lumbar discs has a positive effect on bony fusion [67]. Given these advantages, numerous surgeons have performed PLIF for the treatment of degenerative spondylolisthesis [8910111213].
Many studies have compared these two approaches. However, few reports have focused on a single vertebral level. Indications of these approaches remain unclear. Therefore, the objective of this study was to retrospectively compare the clinical outcomes of PLF and PLIF for degenerative spondylolisthesis of the fourth lumbar vertebra showing predicted instability on plain radiographic evaluation.
Materials and Methods
1. Patients
This study was approved by the ethical committees of two institutions. In these two facilities, PLF and PLIF were performed for 24 and 46 patients, respectively, with degenerative lumbar spondylolisthesis in the fourth vertebra from January 2008 to January 2010. All PLF procedures were performed in Shinshu University Hospital because Nagano General Rehabilitation Center indicated PLIF for all degenerative spondylolisthesis cases during that period. Subjects who underwent single vertebral level (L4–L5) decompression for unstable degenerative spondylolisthesis of the fourth lumbar vertebra with the following conditions and were followed up for ≥2 years were retrospectively studied: Meyerding grade II or more, slip of ≥10° or ≥4 mm in the maximum flexion and extension, and posterior opening of ≥5° in the maximum flexion. Twelve subjects (11 men, 1 women; mean age, 69.5±7.7 years) underwent PLF with a mean follow-up period of 2.8±0.9 years. A total of 19 subjects (7 men, 12 women; mean age, 69.4±6.0 years) underwent PLIF with a mean follow-up period of 2.6±0.7 years. The clinical outcomes were evaluated by performing radiographic imaging. Japanese Orthopaedic Association (JOA) scores were compared between the PLF and PLIF groups.
2. Surgical methods
For the PLF procedure, decortications of the transverse process and local bone graft of the inter-transverse process was performed after posterior decompression and instrumentation with a pedicle screw. Hydroxyapatite granules or beta-tricalcium phosphate granules were added if the harvested local bone was insufficient. For the PLIF procedure, posterior decompression by bilateral total facetectomy and pedicle screw instrumentation was performed. Correction was achieved by using screw depth gap. Disc space curettage procedure was performed by distraction between screws and cutting the vertebral disc to the cartilage endplate. Fixation was performed using local bone grafting, placement of a local bone-filled cage, and addition of a compression force. Cage selection was made according to surgeon's preference.
3. Outcome measurements
1) Clinical evaluation
JOA score and JOA subscores for back pain and walking ability were measured preoperatively, 1, 3, 6 months, 1 year postoperatively and at the final follow up. Improvement rates were calculated by using Hirabayashi's method [14] and JOA scores before surgery, 1, 3, 6 months, 1 year postoperatively and at the final follow-up using the following equation: (postoperative score–preoperative score)/(29 points–preoperative score)×100%. Length of hospital stay, surgical time, intraoperative blood loss, and postoperative blood loss until drainage removal were measured. Postoperative complications were also studied.
2) Radiographic evaluation
Slip length and angle, slip ratio, disc height, and lumbar lordosis (L1–S1) were assessed preoperatively, 1 week, 3 months, 6 months, 1 year postoperatively and at the final follow-up. Slip length and angle were measured by performing radiographic kymography on maximum flexion and extension preoperatively, 3, 6 months, 1 year postoperatively and at the final follow-up.
To control bias caused by the magnification factor on radiographic imaging, slip ratio (% of slip) was measured. Length of the upper endplate of the L5 vertebra was defined as 100%. Disc height was measured as follows: a vertical line was made from the midpoint of the line between the anterosuperior margin of the L5 vertebral body and posteroinferior margin of the L4 vertebral body to the L5 upper endplate. The distance from the endplate to L4–L5 was measured. To correct bias due to radiographic image magnification, the percentage of disc height was calculated as the ratio to L4 posterior vertebral height (H) as follows: disc height%=postoperative disc height (h)/posterior wall height of the L4 vertebral body (H). Lumbar lordosis was evaluated by the angle between the superior edge of the vertebral body of L1 and S1 (Fig. 1).
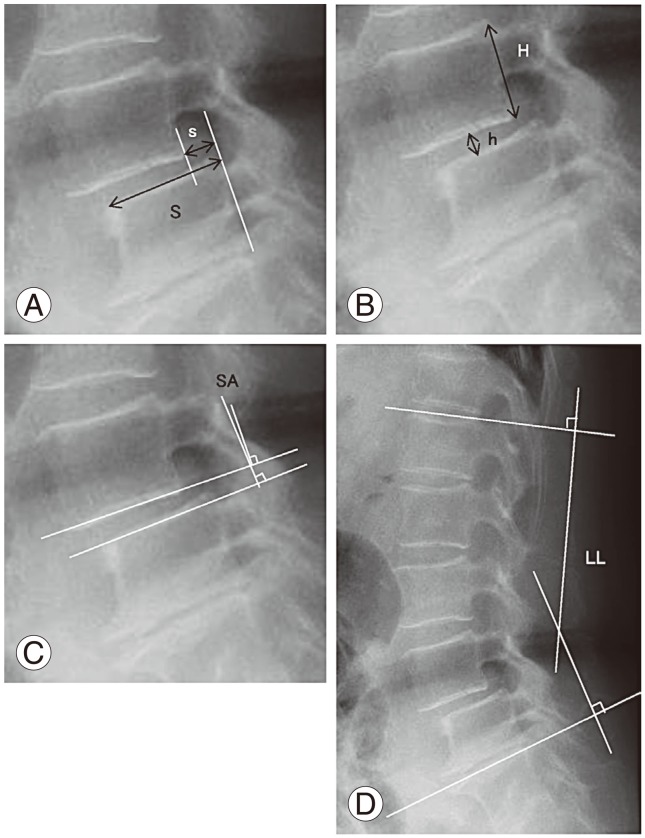
Radiologic measurements of (A) percentage slip, (B) disc height, (C) slip angle, and (D) lumbar lordosis. The SA, degree of slip (% of slip), and disc height (h/H) were measured for the sagittal profile. LL was measured for alignment. S, slippage; h, disc height; H, posterior wall height of the proximal vertebral body; SA, slip angle; LL, lumbar lordosis.
Bony union was evaluated at the final follow-up. Fusion was defined as follows: In PLF, bilateral or unilateral fusion mass was observed between the transverse processes on plain radiograph or CT and radiographic kymography of the maximum flexion and extension revealed a slip angle difference of <4°; In PLIF, bone fusion seen on the radiographs was classified into 4 grades [15]. Grade 1 was defined when a complete fusion achieved with bone bridge formation between the upper and lower vertebral bodies was observed. Grade 2 was defined when bone bridge was not formed but there was no translucency observed around the cages with thick fusion mass formation. Grade 3 was defined when fusion was not achieved with translucency seen around the cages. Grade 4 was defined when cage sunk into the vertebral body or there was bone resorption around cages indicating pseudarthrosis. We aslso measured the standing flexion–extension angle to evaluate mobility. Result was labeled as "achieved fusion" when it was grade 1 or grade 2 with a flexion-extension angle of less than 4° [1516]. It was labeled as "nonfusion" when it was rated grade 3 with flexionextension angle measuring 5° or more. It was labeled as "pseudarthrosis" when it was rated grade 4.
4. Statistics
Student's t-tests were used to compare clinical and radiologic measurements. Fisher's exact test was used to compare the rates of intertransverse fusion. All analyses were performed by using the SPSS ver. 10.0 (IBM Co., Endicott, NY, USA). Statistical significance was considered when p-value was less than 0.05.
Results
1. Clinical results
The age and sex distributions were in the PLIF group were similar to those in the PLF group (Table 1). The JOA scores of the PLF group preoperatively, 1, 3, 6 months, 1 year postoperatively and at the final follow-up were 12.1±5.0, 23.4±2.3, 21.8±4.1, 22.1±3.5, 24.2±3.35, and 23.9±3.1, respectively. Those of the PLIF group were 14.8±5.0, 23.7±1.6, 24.2±2.3, 22.7±4.3, 23.4±5.6, and 24.3±5.3, respectively. There was no significant difference between the two groups. JOA walking function subscores of the PLF group preoperatively, 1, 3, 6 months, 1 year postoperatively and at the final follow-up were 0.8±0.6, 2.2±0.8, 2.6±0.5, 2.4±0.7, 2.7±0.4, and 2.4±0.7, respectively. Those of the PLIF group were 0.6±0.8, 2.1±1.0, 2.8±0.4, 2.2±1.0, 2.4±0.9, and 2.4±1.0, respectively. There was no significant difference between the two groups. The JOA back pain subscores of the PLF group preoperatively, 1, 3, 6 months, 1 year postoperatively, and at final follow-up were 1.3±0.8, 2.0± 0.8, 2.4±0.5, 2.0±0.5, 2.5±0.5, and 2.3±0.7, respectively. Those of the PLIF group were 1.8±0.8, 2.3±0.7, 2.5±0.5, 2.2±0.8, 2.2±0.9, and 2.5±0.6, respectively. There was no significant difference between the two groups (Fig. 2).

(A–D) Clinical data. There was no significant difference in total JOA score, JOA subscore of back pain, or walking ability between PLF and PLIF groups at each follow-up time point. JOA, Japanese Orthopaedic Association; Preop., preoperative; PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion.
The improvement rates according to Hirabayashi's method of the PLF group, 1, 3, 6 months, 1 year postoperatively, and at the final follow-up were 61.0%±29.6%, 59.5%±23.7%, 64.4%±19.7%, 73.5%±17.2%, and 70.4%±19.1%; those of the PLIF group were 64.2%±12.6%, 67.1%±14.1%, 53.0%±35.3%, 60.9%±42.8%, and 68.4%±33.4%, respectively. There was no significant difference between the two groups (Fig. 2).
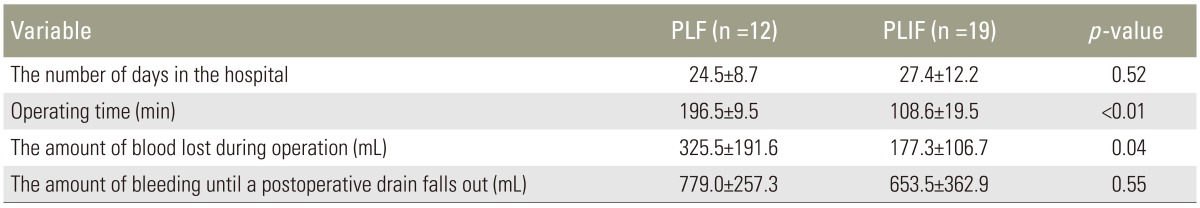
Results of each parameter in the PLF group and the PLIF group were as follows: hospital stay of 24.5±8.7 days and 27.4±12.2 days (p=0.52); surgical time of 196.5±9.5 minutes and 108.6±19.5 min (p<0.05); intraoperative blood loss of 325.5±191.6 mL and 177.3±106.7 mL (p<0.05); and postoperative blood loss until drainage removal of 779.0±257.3 mL and 653.5±362.9 mL (p=0.38). Surgical time and intraoperative blood loss were significantly (p<0.05) decreased in the PLIF group compared to those in the PLF group (Table 2).
The following complications occurred. In the PLF group, there were four major complications, including two permanent L5 injuries, one permanent unilateral blindness, and one transient dermatomal pain that was resolved after one month. In the PLIF group, there were 11 major complications: three deep wound infections, two permanent leg pain, two transient leg pain, one permanent drop foot, one transient drop foot, one deep vein thrombosis, and one pulmonary embolism.
2. Radiographic results
The mean slip angle in the PLF group decreased from 2.9°±7.2° preoperatively to -0.5°±8.9° at the final follow-up. In the PLIF group, it was increased from 6.9°±6.2° preoperatively to 8.9°±5.7° at the final follow-up. This value was significantly (p<0.05) different between the two groups at 3 months postoperatively (Fig. 3A).

(A–D) Radiologic data of the operated segment. Postoperative correction was better and the vertebral disc height was higher in the PLIF group, although the preoperative slip angle and translation were not significantly different between the two groups. Preop., preoperative; PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion; h, disc height; H, posterior wall height of the proximal vertebral body. *p<0.05, **p<0.01.
Preoperative translation (mm) in the PLF and PLIF groups were 7.2±3.9 mm and 5.7±3.0 mm, respectively (p=0.33). At 1 week postoperatively, the values were 6.4±4.1 mm and 1.8±2.6 mm, respectively (p=0.005). From that time point, the correction of translation was in the PLIF group through the final follow-up was significantly better than that in the PLF group (Fig. 3B). The preoperative slip ratios of the PLF and PLIF groups were 24%±11% and 16%±11%, respectively (p=0.09). At 1 week postoperatively, the values were 19%±12% and 4.8±7.1%, respectively (p=0.004). PLIF resulted in significantly (p<0.05) better correction from this time point through the final follow-up (Fig. 3C). Preoperative vertebral disc heights (h/H) of the PLF and PLIF groups were 36%±15% and 30%±9.8%, respectively (p=0.35). At 1 week postoperatively after surgery, the values were 27%±13% and 47%±8.1%, respectively (p=<0.001). The PLIF group showed significantly (p<0.05) higher vertebral disc height from this time point through the final follow-up (Fig. 3D). Preoperative lumbar lordosis (L1–S1) of the PLF and PLIF groups were 38°±15° and 38°±12°, respectively (p=0.70). At the final follow-up, the values were 27°±24° and 37°±7°, respectively (p=0.12). There was no significant difference between the two groups (Fig. 4).
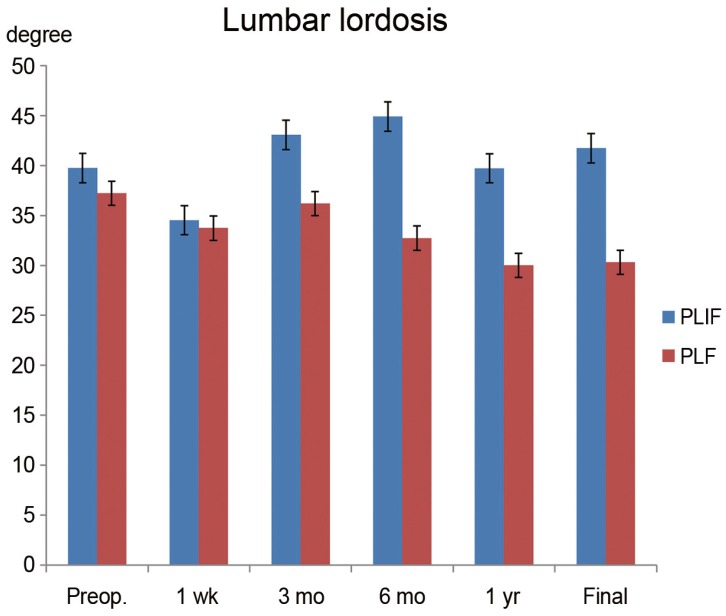
Radiologic data for the sagittal alignment. Lumbar lordosis was not significantly different between the two groups. Preop., preoperative; PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion.
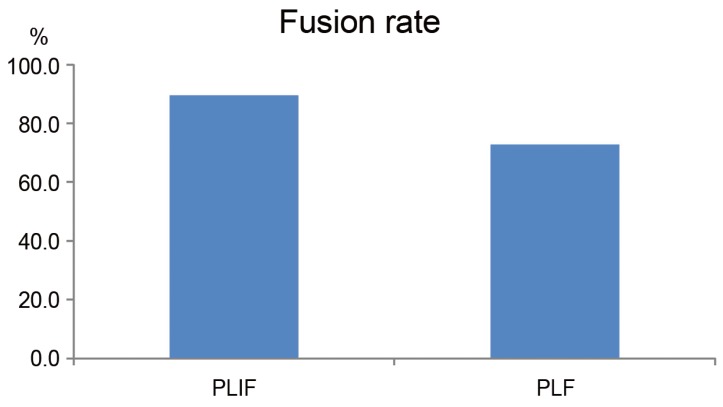
The preoperative slip angles on maximum flexion and extension in the PLF and PLIF groups were 8.0°±5.3° and 12.0°±5.1°, respectively (p=0.04). The PLIF group was significantly unstable. However, at 3 months postoperatively, the values were 5.7°±3.4° and 1.9°±2.5°, respectively (p=0.02). At 6 months postoperatively, the values were 3.9°±2.3° and 1.5°±1.7°, respectively (p=0.02). Thus, the slip angle of the PLIF group was more limited. However, at the final follow-up, the slip angles of the PLF and PLIF groups were 3.3°±1.6° and 3.1°±2.4°, respectively (p=0.8) (Fig. 5A). Preoperative translation on maximum flexion and extension in the PLF and PLIF groups were 2.3±1.4mm and 3.9±2.5 mm, respectively (p=0.1). At 3 months postoperatively, the values were 2.2±1.3 mm and 0.9±1.0 mm, respectively (p=0.04). Thus, the PLIF group showed significantly limited translation in motion. However, there was no significant difference at 6 months postoperatively or at the final follow-up (PLF and PLIF group: 2.0±1.8 mm and 1.6±1.7 mm, respectively, p=0.5) (Fig. 5B). The fusion rates of the PLF and PLIF groups were 72.3% and 89.5%, respectively (p>0.05) (Fig. 6).
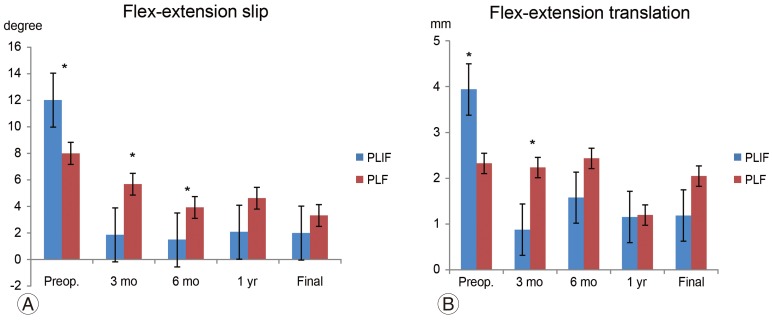
(A, B) Radiologic data on maximum flexion and extension. Preoperative instability on maximum flexion and extension was stronger in the PLIF group. However, the PLF group showed instability at 3 and 6 months postoperatively compared to the PLIF group. The instability in the maximum flexion and extension disappeared in both groups. Preop., preoperative; PLIF, posterior lumbar interbody fusion; PLF, posterior lumbar fusion. *p<0.05, **p<0.01.
Discussion
The theoretical advantages of PLIF include anterior column support, indirect foraminal decompression, restoration of lordosis, and maintenance of intervertebral disc height [17]. Anterior column augmentation in the PLIF procedure for degenerative spondylolisthesis, which employs autologous iliac bone graft in addition to pedicular screw fixation and PLF, is likely to achieve better fusion rate and clinical results [18]. Crawford et al. [19] reproduced grade I lumbar spondylolisthesis using cadaveric specimens and studied the biomechanics of various instrumentation combinations, including cages with or without intersomatic spacers, pedicle screws alone, and pedicle screws with cages. Pedicle screws with cages presented better biomechanics in flexion, lateral extension, axial rotation, and shear force. Specimens with cages showed instability and fatigue. They recommended the use of screw systems and cages in grade I lumbar spondylolisthesis patients because greater stability might allow good fusion around the cages.
Suk et al. [13] recommended the PLIF procedure because PLF was associated with more cases of pseudoarthrosis while PLIF has preferable characteristics such as anterior column support, maintenance of reduction, and a better fusion rate. Kim et al. [20] compared PLF, PLIF, and the combination the two and found that PLIF had better surgical time, blood loss, and donor site pain, although the clinical results of the three were similar. In our study, the PLIF group showed significantly shorter surgical time. However, as PLIFs were performed by two experienced doctors in one facility while PLFs were mainly performed in a university hospital by two senior spine surgeons and 4 fellows, learning curve might have influenced the result. Musluman et al. [21] reported that PLIF could achieve better sagittal balance and that the back pain visual analogue scale (VAS) scores were improved from the early postoperative period to the final follow-up. However, some authors have reported that the clinical results of PLIF are not superior than PLF in the treatment of low-grade spondylolisthesis [20212223], although PLIF is expected to achieve better maintenance of correction and bony union.
Most previous reports compared cases of spondylolysis/spondylolisthesis or multi-vertebral level. Few studies compared the results at a single vertebrae level. Ha et al. [18] compared PLF and PLIF for degenerative lumbar spondylolisthesis at single level at L4/5 in regard to clinical outcomes and radiographic parameters. They classified subjects into a stable group and an unstable group using a degree of slip threshold of 4 mm and a slip angle of 10°. The clinical outcomes in the stable group for PLF and PLIF were similar to each other. However, PLIF resulted in better clinical outcomes in the unstable group. They also reported that PLF in unstable group without anterior support showed higher possibility of sagittal imbalance caused by disc space re-narrowing, progression of displacement, loss of reduction, or non-union. However, in their study, the vertebral disc height increased in both stable and unstable groups, while the degree of slip, lordosis angle, and sacral tilt was not affected by insertion of the cage. In the present study, PLIF and PLF for unstable degenerative spondylolisthesis were compared. We found that their clinical results were not significantly different. Subjects with greater instability were defined by degree of slip ≥4 mm, slip angle ≥10°, posterior opening on maximum flexion ≥5°, and Mayerding grade ≥II. However, some subjects with a degree of slip <4 mm and slip angle <10° were also included in this study. In this study, PLIF showed better results in the correction of slip, maintenance of vertebral disc height, and fusion rate. However, lumbar lordosis in the PLIF group was not better than that in the PLF group, which might be due to the lack of difference in clinical outcomes between the two groups.
Some authors consider PLIF procedure difficult due to increased bleeding, prolonged operation time, and more extensive dissection [1524252627]. Complications associated with the PLIF procedure include permanent neurological deficit (0.4%–1.7%), cerebrospinal fluid leakage (0.4%–0.5%), radicular pain (1.1%–2.5%), posterior cage dislocation (0.8%–0.9%), and deep wound infection (0.6%–5%) [118282930]. Musluman et al. [21] have reported that in PLIF, complications due to nerve root retraction are found in only one patient (4%). Those complications are not permanent. This low rate of neurological deficits may be attributed to the insertion of PLIF cages in a manner similar to the transforaminal lumbar interbody fusion approach after bilateral facetectomy and laminectomy, which can minimize nerve root retraction. The average bleeding volume in the PLF group was 270 mL, which was greater than that in the PLIF group. In the PLF group, there were 4 major complications (2 permanent L5 injuries, 1 patient with permanent unilateral blindness, and 1 patient with transient dermatomal pain, which resolved after 1 month). In the PLIF group, there were 11 major complications (3 deep wound infections, 2 patients with permanent leg pain, 2 with transient leg pain, 1 patient with permanent and 1 with transient drop foot, 1 patient with a deep vein thrombosis, and 1 patient with a pulmonary embolism). The higher number of complications in the PLIF group might have resulted from a more invasive nature of total facetectomy or discectomy. In contrast, PLF employs in situ fixation. Although the PLIF group showed a better slip correction rate, root injury might have occurred during correction. The limitations of this study include the following. First, multiple surgeons in the two facilities performed the surgeries. Second, the case number used in this study was small. Third, the follow-up rate in the PLF group was low. Fourth, clinical evaluation in this study was not performed by patients-oriented measure.
Conclusions
PLF was compared to PLIF for patients with unstable L4 spondylolisthesis. Clinical outcomes by JOA scores were not significantly different between the two groups. Post-fixation correction was better in the PLIF group. However, no significant difference was observed for lumbar lordosis between the two groups.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.