Outcomes after Surgery for Spinal Metastasis of Colorectal Origin: Case Series
Article information
Abstract
Study Design
Retrospective study.
Purpose
The aim of this study was to evaluate the clinical management and outcomes of patients who underwent surgical intervention for metastatic colorectal adenocarcinoma of the spine.
Overview of Literature
Gastrointestinal (GI) cancer metastasis to the spine are relatively rare and represent later manifestations of the disease. Studies and reports on the outcomes of patients who undergo surgery for spinal metastasis of GI origin are scarce.
Methods
A retrospective chart review of all patients who underwent surgery for spinal metastasis of colorectal origin was performed. Four patients were identified. Patient characteristics, outcomes, and survival were analyzed.
Results
Two patients experienced improvement in pain or myelopathic symptoms. Although the mean survival was 15.3 months, this average included a patient still living at 57.1 months. The mean survival was just 1.3 months for the 3 patients who expired.
Conclusions
In certain cases, symptomatic improvement with prolonged survival is possible after surgery for metastatic spinal lesions of colorectal origin; however, survival is poor in the majority of cases.
Introduction
Metastases represent the majority of spinal cancers [1]. The primary concern with metastasis is pathologic fracture and/or spinal cord compression, which may lead to intractable pain, sensory alterations, weakness, and/or paralysis [2,3,4,5,6,7,8]. Operations in these patients are palliative and are typically performed for symptomatic relief, and thereby, to improve the quality of life rather than survival [2,3,4,5,6,7,8]. Of all the metastatic spinal lesions, those of gastrointestinal (GI) origin are particularly aggressive, with the mean survival as low as 2.6 months after diagnosis of a GI-primary spinal lesion [2,9], and high recurrence rates relative to metastases of other primary cancers [10]. These tumors are also particularly rare, making them difficult to study. GI cancers that reportedly metastasize to the spine include: colon cancer [1,11,12,13,14,15,16], hepatocellular carcinoma [3,17,18,19,20], gastric cancer [1,10], and more rarely, cholangiosarcoma [21,22,23,24]. There is a paucity of data available regarding the overall management of patients with GI spinal metastasis, and even less regarding surgical management and outcomes. In this article, we report our findings from a series of 4 surgically managed patients with metastatic spinal disease from colorectal adenocarcinoma and review the current literature on the subject.
Materials and Methods
Following approval by the University of Michigan Institutional Review Board, we queried the electronic medical records for all adult patients who underwent surgery for spinal metastasis from colorectal cancer for the period of 2005 to 2011. Patients were considered candidates for surgery if symptoms included refractory pain and/or neurologic deficit, concern for instability, and if life expectancy was predicted to be at least 3 months. A total of 4 patients underwent spinal surgery. In each case, tumor pathology confirmed metastatic colorectal cancer. A number of covariates in each patient's case were reviewed: age, sex, presenting symptomology, primary tumor site, presence of extraspinal metastases, comorbidities, use of adjuvant therapies (chemotherapy and/or radiotherapy), procedure performed, functional status (measured by Karnofsky Performance Score [KPS]), neurological status (measured by Nurick score), and pain (measured by visual analogue scale [VAS]). Perioperative and postoperative characteristics of interest included estimated blood loss and complications. Complications were defined as procedure-related or unexpected events, requiring additional intervention at any time within 30 days following the surgery. Clinical outcomes included the Medical Research Council scale of 0 to 5 [25] to assess motor strength, Nurick score [26] of 0 to 5 to assess myelopathy, and follow-up KPS. Survival length was recorded. Recurrence and repeat surgery for recurrence were also documented.
Results
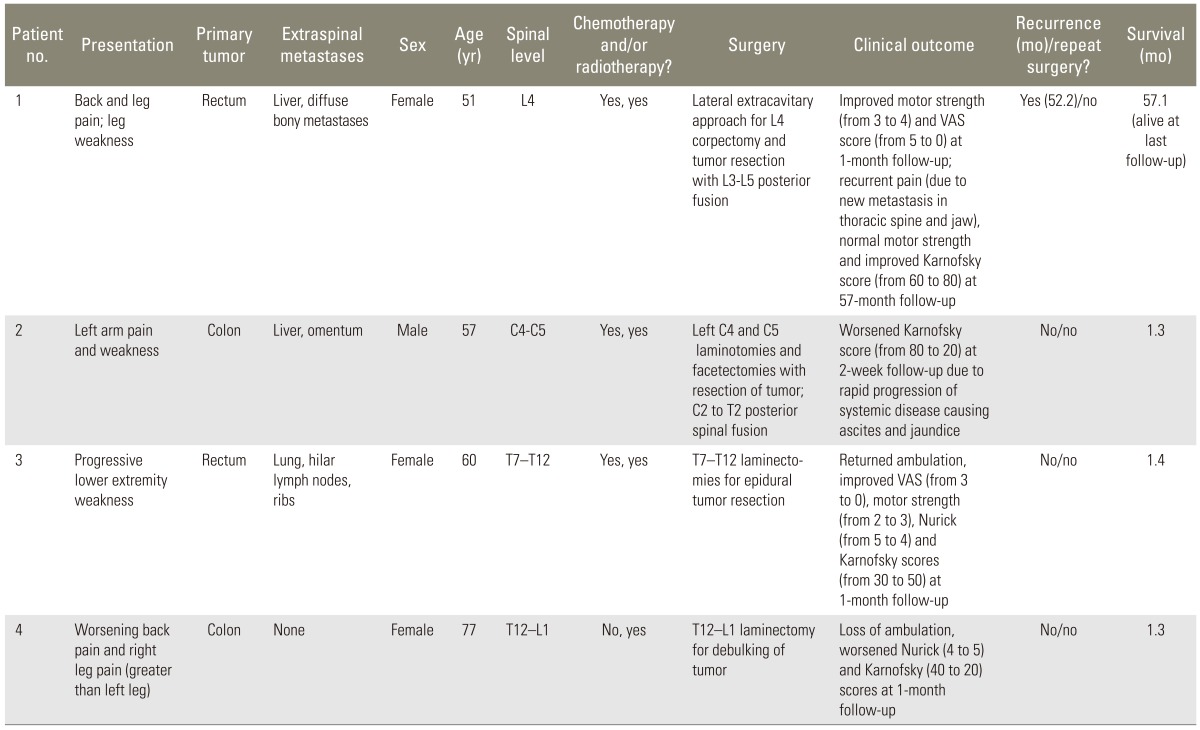
Table 1 displays demographic information, clinical details, and outcomes for each of the 4 patients included in the study. The mean age at the time of surgery was 61.3 years for colorectal patients, and 1 patient (25.0%) was male. Of the 4 patients, 1 was presented with the inability to ambulate due to lower extremity weakness, 1 presented with back pain only as a primary symptom, and 2 presented with both pain and weakness. Metastases were located in the cervical spine in 1 patient, thoracic spine in 2 patients, and lumbar spine in 1 patient. Surgical procedures are also detailed in Table 1 (Fig. 1). The mean estimated blood loss was 762.5 mL.

(A) Sagittal T2-weighted magnetic resonance imaging of patient #1 showing L4 metastasis causing spinal stenosis. (B) Anteroposterior and (C) lateral X-rays showing interbody cage placement and posterior instrumentation after posterolateral corpectomy for tumor resection.
Postoperatively, 2 patients had no change in neurological status, while 1 patient regained ambulatory ability for an improved Nurick score, and another experienced loss of ambulation with a decline in the Nurick score. Two patients (patients #1 and #3) showed improved KPS and strength, while the other 2 had lower KPS and no strength changes. Of the 3 patients with pain, 2 experienced significant pain relief with at least a 2-point improvement in VAS. Recurrence occurred in 2 patients, with 1 of the 2 undergoing repeat surgery for worsening pain and neurologic deficit 1 month after the initial operation. Although the overall mean survival was 15.3 months, this average included a still-living patient. The mean survival of patients who expired was just 1.3 months.
Discussion
About 1.2 million cases of colorectal cancer are diagnosed globally each year, with most being adenocarcinomas [27]. Although more aggressive than breast cancer, mortality rates are otherwise lower than for solid tumors [28]. There is little evidence concerning the surgical management of spinal metastases of colorectal primary origin. Currently, the overall mean survival for all spinal metastases following surgery is about 7 to 8 months [2,17,29,30], with metastasis from GI origin associated with one of the lowest mean survivals at 2.6 months [2]. Patients with metastases from rectal primary sites have been shown to have significantly improved survival over patients with colon primary tumors (7.9 months and 2.7 months, respectively) [31]. Osseous metastases from colorectal malignancies are infrequently seen [11], usually representing a late manifestation of the disease [12], and are frequently asymptomatic [15]. Incidence of such metastases is reported to range anywhere from 2% to as high as 24% in autopsy cases [11,12,13,14,16]. Overall, it has been reported that colorectal adenocarcinoma accounts for 1.5% to 4.7% of all metastatic spinal disease [6,31,32]. Colorectal cancer bony metastases are primarily osteolytic and most often involve the vertebral column [33]. The main sites of spinal metastases are the lumbar (36% to 75%) and thoracic spine (17% to 61%), followed by the sacral (6% to 35%) and cervical vertebrae (2% to 7%) [13,34,35]. In about 16% of patients with spinal metastasis, pathological fracture and/or spinal cord compression are found [33].
Medical management of colorectal metastases most commonly consists of chemotherapy with 5-fluorouracil and leucovorin, oxaliplatin or irinotecan, and bevacizumab, cetuximab, or panitumumab where indicated [36,37,38,39,40]. With recent improvements in medical management, survival following colorectal metastasis has been reported to be greater than 20 months. However, this increase in survival has subsequently led to an increase in bony metastasis and its clinical manifestations in these patients. In cases where the patient experiences intractable pain and/or neurological compromise, which causes significant debilitation and overall dysfunction, surgery is a consideration.
The main goals and advantages of surgery in patients with spinal metastasis include decreasing tumor burden, pain relief, prevention and reversal of neurologic deficit, and/or preserving and restoring spinal stability [10,41]. However, there are very few case reports and only 2 small case series that exclusively evaluated surgical management of these patients. In a study focusing primarily on radiotherapy, Brown et al. [31] followed 3 patients who were treated surgically and noted that survival remained poor with patients dying at 3, 4, and 9 months, postoperatively. In that entire cohort, the mean overall survival was 4.1 months, which was similar to other reports of patients who were medically managed [31,33,42]. However, the surgically managed patients tended to fare better than those treated non-operatively; the median overall survival for those 3 patients was 5.3 months [31]. In addition, all 3 surgically treated patients remained ambulatory until death [31]. There is also evidence that more aggressive surgical management can offer prolonged survival. Chen et al. [43] reported an improved mean survival of 10 months with more aggressive treatment, especially in patients with neurological deficit.
While survival time for our colorectal cohort was relatively long with a mean of 15.3 months, the average is biased due to a still-living patient with a survival time of 57.1 months at time of the latest follow-up. Thus, long-term survival is possible in these patients, though our data seem to indicate it to be the exception rather than the rule.
Radiotherapy and/or chemotherapy show limited benefit as the sole means of therapy, especially for metastasis of the GI origin, as several studies have shown that few non-ambulatory patients with metastatic spinal disease of colorectal origin regain ambulation after treatment with chemo-radiotherapy alone [6,31,44,45,46]. As adjuvant therapy, however, these modalities hold an important place in the treatment of these patients [15]. For patients with short predicted survival or patients unable to tolerate surgery, vertebroplasty and radiotherapy are alternatives [17].
Conclusions
There are no established methods of treating colorectal spinal metastasis, and there are few reports and recommendations regarding this topic. From our experience and available data, surgical resection and stabilization can result in pain relief, improved neurological function, and improved quality of life. Special care should be taken when assessing patients with colorectal spinal metastases as candidates for surgery, however, as most of these patients have a relatively poor prognosis.
Notes
No potential conflict of interest relevant to this article was reported.