Cervical Ossification of Posterior Longitudinal Ligament in X-Linked Hypophosphatemic Rickets Revealing Homogeneously Increased Vertebral Bone Density
Article information
Abstract
There is no report that describes in detail the radiological and intraoperative findings of rickets with symptomatic cervical ossification of the posterior longitudinal ligament. Here, we describe a case of X-linked hypophosphatemic rickets with cervical ossification of the posterior longitudinal ligament presenting unique radiological and intraoperative findings. The patient presented progressive tetraparesis. Magnetic resonance imaging studies revealed severe cervical spinal cord compression caused by ossification of the posterior longitudinal ligament. Computed tomography scans revealed homogeneously increased vertebral bone density. An expansive laminoplasty was performed. At surgery, homogeneously hard lamina bone was burdened in drilling and opening of the laminae. The patient's neurological symptoms were improved postoperatively. Bony fusion of the hinges occurred postoperatively. Therefore, expansive laminoplasty could be performed for symptomatic cervical ossification of the posterior longitudinal ligament with X-linked hypophosphatemic rickets. However, unusual bone characters should be taken into consideration for careful operation during surgery.
Introduction
X-linked hypophosphatemic rickets (XLHR) is characterized by X-linked dominant pattern of inheritance and hypophosphatemia associated with decreased renal tubular reabsorption of phosphate [1]. Spinal canal stenosis and spinal cord compression caused by thickening of the laminae, hypertrophy of the spinal ligaments, or facet joints in XLHR [2] are rare. Here we report a case of XLHR with severe cervical spinal cord compression caused by ossification of the posterior longitudinal ligament (OPLL) with unique radiological and intraoperative findings.
Case Report
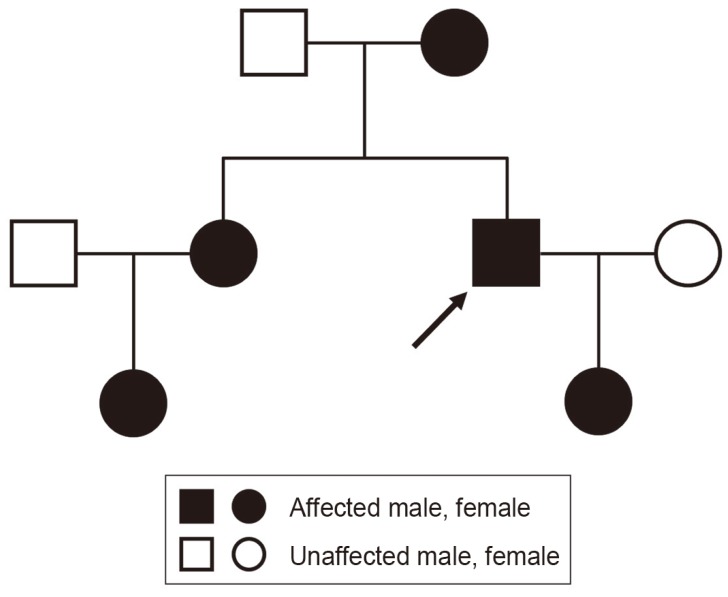
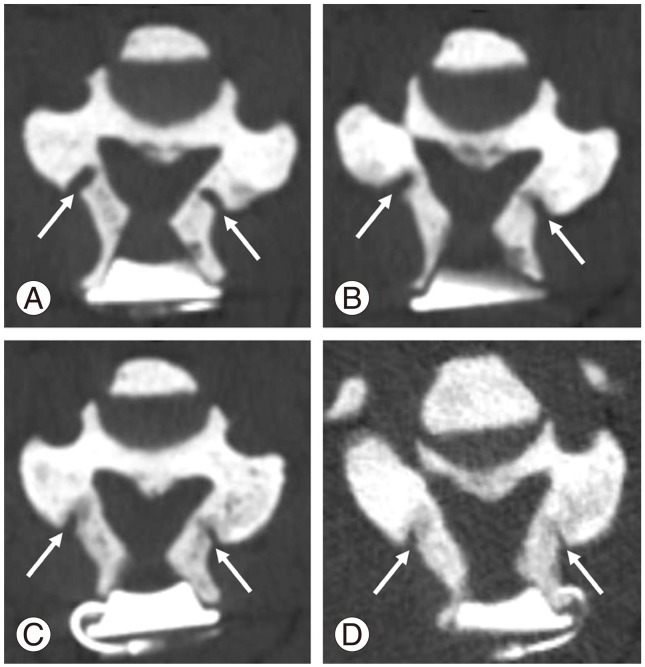
A 32-year-old man was referred to our hospital due to a 1-year progressive sensorimotor disturbance to the extremities. He had been diagnosed with vitamin D-resistant rickets and received 1α-hydroxyvitamin D3 [1α-(OH)-D3] (1 µg/day) since his childhood. His mother, older sister, nephew and daughter also had rickets (Fig. 1). He was of short stature (151.0 cm) with limb shortening. Neurological examination revealed numbness below T4, weakness (manual muscle testing, 4/5; grip strength: right 17.0 kg, left 13.0 kg), deep tendon hyper-reflexes of the upper and lower extremities, and spastic gait disturbance needing assistance, comparable to the Japanese Orthopaedic Association (JOA) score [3] of 7 points. His serum phosphate level (2.0 mg/dL; normal range, 2.3-4.5 mg/dL) was low, whereas his alkaline phosphatase (726 units/L; normal range, 100-350 units/L) and high sensitivity parathyroid hormone levels (770 pg/mL; normal range, 160-520 pg/mL) were high. His serum levels of calcium, intact-parathyroid hormone, growth hormone, insulin-like growth factor, 125-hydroxyvitamin D3, and 1, 25-dihydroxyvitamin D3 [1, 25-(OH)2-D3] were all within normal ranges. Plain radiography and computed tomography (CT) of the cervical spine demonstrated a mixed type of OPLL from C2 to C7 (Fig. 2A-C). Magnetic resonance imaging (MRI) demonstrated cervical spinal canal stenosis and spinal cord compression at the C2/3 to 5/6 levels. The compression was prominent the most at the C4/5 level with intramedullary high signal intensity on T2-weighted images (Fig. 2D). CT also demonstrated generalized bone density increase, mimicking osteopetrosis (Fig. 2A-C). Although a genetic examination was not performed, a diagnosis of XLHR was made based on his family history, a predominance of females affected, hypophosphatemia, and normocalcemia. Cervical myelopathy due to OPLL was treated with expansive laminoplasty.
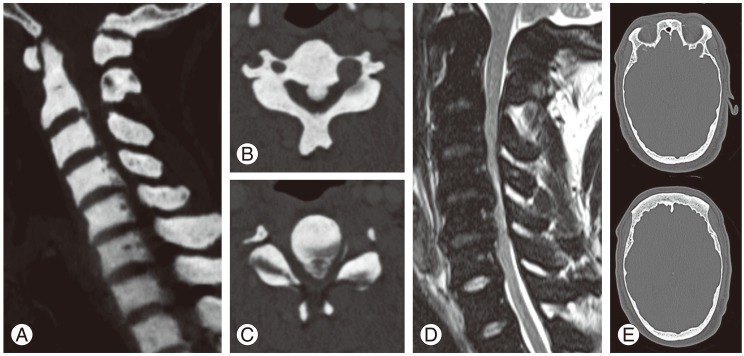
Preoperative computed tomography (CT) scan demonstrating a cervical ossification of the posterior longitudinal ligament (A). Mass of the ossification of the posterior longitudinal ligament is more prominent at the C4 (B) to C4/5 levels (C). T2-weighted magnetic resonance imaging demonstrating spinal canal stenosis and spinal cord compression (D). Axial section of head CT scan demonstrating a thinned cranial bone (E).
The patient was placed in the prone position. His head was fixed with protective helmet system (Prone View, Dupaco, San Diego, CA, USA) which holds the cushion molding a head/face, because his cranial bone was too thin on CT to be fixed with a head-pin frame (Fig. 2E). C2 dome-like laminotomy as well as C3-6 mid-sagittal splitting and lateral guttering were performed using high-speed 2-4 mm-diameter diamond burr under the surgical microscope. His lamina bones were homogeneously hard. Characteristic of cancellous bone was not observed. Thus, the inner cortex was thinned carefully according to the evaluation and measurement by preoperative images. The split laminae were opened at the hinge with unusual strong elasticity in a sequence like a French door using a laminar spreader. After dural pulsation of the cord was observed, hydroxyapatite spacers (APACERAM, PENTAX, Tokyo, Japan) were inserted between the laminae from C3 through C6 using flexible titanium cables (Sof' wire system, Codman, Raynham, MA, USA) (Fig. 3).
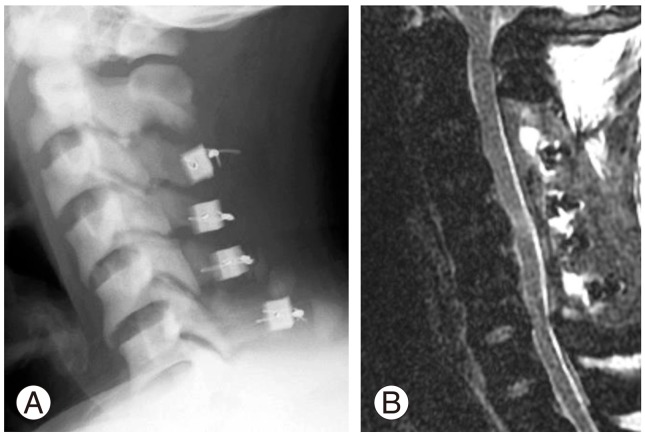
Postoperative plain radiograph (A) and T2-weighted magnetic resonance imaging (B) demonstrating adequate expansion of the spinal canal and decompression to the spinal cord.
He was treated with oral phosphate supplementation since the time of the operation in addition to taking 1α-(OH)-D3 (1 µg/day). Six months after the operation, his sensorimotor disturbance disappeared. His grip strengths were 29.0 kg bilaterally. He could go up and down stairs independently with a clutch (JOA score, 14 points; the recovery rate, 57.1%). CT scans revealed progressive bony fusion of the hinges of expanded laminae at 4, 7, and 14 months after the operation (Fig. 4).
Discussion
XLHR is caused by a loss-of-function mutation in the PHEX gene (phosphate regulating gene with homologies to endopeptidases on the X chromosome) located on X chromosome Xp22.1. XLHR is characterized by inadequate mineralization of bone [4] due to high incidence of hyperparathyroidism [5]. Marked shortening of legs in XLHR might be due to the reduction of mineral content in the peripheral bone [5]. In contrast to peripheral bones, cancellous bone density and mineralization are increased in axial bone, including lumbar spine [5]. However, there is no report of a case exhibiting extremely predominant increase in cancellous bone density of the cervical spine on plain X-rays and CT scans in adult XLHR with cervical OPLL as seen in the present case. The cranial bone thinning observed in this case might have been caused by inadequate bone mineralization in XLHR.
Intraoperative findings in previous reports revealed thickened and hard lamina [6,7], ossified ligamentum flavum and posterior longitudinal ligament [8], and heavily calcified anulus fibrosus [2]. The lamina bones were also hard in this present case. However, intraoperative findings revealed that homogeneously mineralized bone was continued through the lamina and that the characteristic of cancellous bone was absent. In developmental spinal canal stenosis, the cortical bone exists as an obvious different structure in the spinal canal side after the cancellous bone of the lamina is drilled out. In rickets as shown in the present case, the boundary between cortical and cancellous bones might be unclear. Thus, drilling with diamond burr and opening of the lamina were performed in a meticulous fashion. Although there was no mention of using a microscope in previous reports [2,6,7,8], we consider that it is essential to use a microscope during drilling of the lamina to prevent injury to the facet joints and the spinal cord. As to the method of laminoplasty, it is currently unknown whether mid-splitting or open door method is better for our case. We selected mid-splitting laminoplasty because we were more familiar with it. There was no report on open door laminoplasty for cervical OPLL in XLHR.
The bone bonding rate of hinges of the expanded laminae with hydroxyapatite ceramic spacers has been reported to be 84% at 6 months and 98% at 1 year postoperatively in cased of developmental spinal canal stenosis [9]. However, the rate in XLHR has not been reported. Both activated vitamin D and phosphate are recommended for affected adults with spontaneous insufficiency fractures and pending orthopaedic procedures to reduce recovery time [10]. This study showed that bony fusion of the hinges occurred even in XLHR patient who received proper oral phosphate supplementation and vitamin D treatment.
In conclusion, although spinal cord compression with cervical OPLL is a rare complication in XLHR, surgery is a treatment of choice for symptomatic patients. During surgery, unusual bone characters should be taken into consideration for careful operation.
Notes
No potential conflict of interest relevant to this article was reported.