|
 |
- Search
| Asian Spine J > Volume 9(1); 2015 > Article |
Abstract
Purpose
To assess differences in computed tomography (CT) imaging parameters between patients with cervical myelopathy and controls.
Overview of Literature
There is a lack of information regarding the best predictor of symptomatic stenosis based on osseous canal dimensions. We postulate that smaller osseous canal dimensions increase the risk of symptomatic central stenosis.
Methods
CT images and medical records of patients with cervical myelopathy (19 patients, 8 males; average age, 64.4±13.4 years) and controls (18 patients, 14 males; average age, 60.4±11.0 years) were collected. A new measure called the laminar roof pitch angle (=angle between the lamina) was conducted along with linear measures, ratios and surrogates of canal perimeter and area at each level C2-C7 (222 levels). Receiver-operator curves were used to assess the diagnostic value of each. Rater reliability was assessed for the measures.
Results
The medial-lateral (ML) diameter (at mid-pedicle level) and calculated canal area (=anterior-posterior.×ML diameters) were the most accurate and highly reliable. ML diameter below 23.5 mm and calculated canal area below 300 mm2 generated 82% to 84% sensitivity and 67% to 68% sensitivity. No significant correlations were identified between age, height, weight, body mass in dex and gender for each of the CT measures.
Conclusions
CT measures including ML dimensions were most predictive. This study is the first to identify an important role for the ML dimension in cases of slowly progressive compressive myelopathy. A ML reserve may be protective when the canal is progressively compromised in the anterior-posterior dimension.
Cervical spinal stenosis is a multi-factorial process, which can lead to spinal cord compression and eventually myelopathy. Stenosis may be congenitally present, but is most commonly acquired. The primary pathology in congenital stenosis, sometimes referred to as developmental spinal stenosis, is a skeletal hypoplasia in which the dimensions of the cervical canal are reduced. Conversely, an acquired spinal stenosis occurs in response to degenerative changes, which most commonly originate at the disc space level. These two pathologies are often found in tandem and they work synergistically to compress the spinal cord and produce clinical symptoms.
Cervical spinal stenosis is a common condition, occurring most frequently in the sixth decade of life [1]. It is a multi-factorial process, the result of a reactive hypertrophy of the osseous (uncal and endplate osteophytes) and ligamentous structures in conjunction with bulging and/ or failure of the disk space. The end result is a restriction of the anterior-posterior (AP) dimension of the spinal canal and compression of the cervical spinal cord. In 1971, Turnbull [2] postulated that AP compression and limited lateral column arteriolar plasticity lead to cervical myelopathy.
Payne and Spillane [3] measured the AP straight line distance or diameter of the cervical spinal canal as a threshold indicator for spinal stenosis [4]. Anatomy dissections and imaging studies have shown that the human adult spinal cord averages 5 to 6 mm in the AP diameter [5]. Additionally, the intracanicular soft tissue components (posterior longitudinal ligament, dura and ligamentum flavum) occupy another 2 mm on either side of the spinal cord. Thus, the minimal space available for the spinal canal to encase the spinal cord without compressing the structures is 10 mm. This has been set as the threshold value for diagnosing a critical or absolute cervical spinal stenosis. In extension, the spinal canal AP diameter reduces 2 to 3 mm, leading to 12 to 13 mm as a threshold value for relative stenosis [6]. In comparison, the normal adult AP diameter is 17 to 18 mm.
While simple to measure, linear distances, which have been typically measured on plain radiographs, are subject to magnification error. In response to this limitation, several cervical spine geometric parameters have been described which use ratios to negate the impact of magnification. The Pavlov-Torg ratio [7,8] was originally used to discriminate football players at risk for acute spinal cord injury (SCI) and has been proven to be an inconsistent predictor of symptomatic compressive myelopathy [8,9,10,11]. The Matsuura ratio predicts an acute SCI due to cervical fracture and dislocations, based on the ratio of the AP and medial-lateral (ML) dimensions [12]. Its applicability to an atraumatic compressive myelopathy is unknown.
Since the skeletal dimensions of the spinal canal dictate the starting space available for the spinal cord, it is postulated that patients with congenitally small canals are more likely to develop clinical evidence of spinal cord compression in response to normal age-related acquired degenerative changes [3,6]. We sought to identify a measure of the bony canal dimensions that reliably differentiates patients with and without clinically diagnosed cervical myelopathy. This study examines several geometric parameters of the cervical canal, which may help to distinguish between asymptomatic patients and those who have developed a cervical spondylotic myelopathy. We hypothesized that the laminar roof pitch (LRP) angle would more accurately discriminate between patients with and without myelopathy than previously reported measures, like the computed tomography (CT) Pavlov-Torg ratio, the Matsuura ratio and the sagittal AP canal diameter.
This is a retrospective comparative study. It was approved by the Institutional Review Board of the Emory University (Atlanta, GA, USA). Two patient cohorts were identified as follows: (1) controls and (2) myelopathic patients. Controls were identified by screening the Grady Memorial Hospital trauma registry for patients (older than 50 years of age, i.e., in their "6th decade of life") who underwent cervical CT scan with sagittal and coronal reconstructions as part of a negative trauma work-up, that is they had no acute fractures or canal altering changes resulting from their accident. Patients were screened for signs and symptoms or diagnoses of myelopathy or radiculopathy. Subjects were included in the control cohort (="controls"), if their cervical CT scan was negative and there was no clinical evidence of (pre-) existing cervical myelopathy or radiculopathy. Thus, controls were selected from those without a symptomatic cervical spine stenosis who underwent the radiation exposure of a CT scan to the cervical spine due to study unrelated causes. Patients were excluded if medical records and/or imaging studies were not available, if these records indicated the patient had a myelopathy or myeloradiculopathy, if a history of cervical spine surgery or fracture was noted, an ossification of the posterior longitudinal ligament (OPLL) was present or if image quality or cervical alignment precluded the data collection. No radiograph review review was included in this study as this imaging modality was rarely used in the setting of a trauma work-up wherein a CT scan was obtained in a routinely fashion.
The "myelopathic" cohort has been obtained from the clinical database of a single spine surgeon's university practice. Patients with a known diagnosis of myelopathy or myeloradiculopathy were included. Myelopathy was diagnosed in patients with at least 3 of following symptoms: decreased upper extremity dexterity, diffuse sensory alteration (non-dermatomal), gait instability, diffuse subjective weakness (non-myotomal), bowel and/or bladder dysfunction, hyperreflexia, Hoffman's sign and Lhermitte's sign [13]. Radiculopathy was defined by neck pain radiating in a specific nerve root distribution, possibly associated with motor, sensory or reflex disturbances in the same distribution. Radiculopathy alone was not sufficient for inclusion. Patients were excluded if their medical records and/or imaging studies were not available or did not clearly indicate the disease status, an OPLL was present, a history of cervical spine surgery or fracture was noted or if the image quality or cervical alignment precluded an accurate measurement.
Axial (mid-pedicle level) and midline sagittal CT images were used for measurements in all subjects. The definitions of the measures are summarized in Table 1. On sagittal images, measurements included the (1) AP canal diameter measured at the mid-height level of the vertebra and (2) the canal diameter at the disc level (CDDL); (3) AP diameter of the vertebral body (VB); (4) the CT Pavlov-Torg ratio [14,15](=canal diameter /VB) and (5) the CT Pavlov-Torg ratio measured at the disc space (=CDDL/VB) (Fig. 1A). On axial images, measurements included the (1) LRP angle and the (2) Matsuura ratio, which is the AP diameter of the canal at a mid-pedicle level divided by the ML canal diameter at the same level 13 (Fig. 1B). The LRP was the novel measure tested in this study. It is the interior (i.e., pointing toward the canal) angle subtended by two lines drawn along the axis of the left and right cervical lamina, measured on the axial images at the mid-pedicle level (red lines in Fig. 1B). Measurements were performed on the second through seventh cervical vertebrae (C2-7) for all subjects.
Sensitivity and specificities of LRP angle, Pavlov-Torg ratio, Matsuura ratio, sagittal AP canal diameter and transverse ML canal diameters as well as calculated surrogates for canal perimeter and area using linear values as described above were evaluated by receiver operator curves. A subject was considered test-positive if any vertebral level (C2-C7) was sub-threshold (i.e., ML<23.5 mm). A two-way analysis of variance was performed to identify significant differences using cohort and vertebral level as factors followed by Tukey's posthoc procedure [16]. An univariate correlation analysis was performed between age, height, weight, body mass index, gender and all geometric parameters in the control group and the myeloradiculopathy group to evaluate the impact of gender and morphometric data. Pearson's and Spearman's coefficients were calculated. Significance was attributed to a p-values less than 5%. Lastly, we assessed the intra-rater and inter-rater reliability of the tested measures. Two fellowship-trained spine surgeons on two separate occasions measured each of the 4 fundamental parameters (canal diameter, VB, LRP, ML) used to achieve the raw and composite measures analyzed in this study at the C2-C7 levels on 10 patients (5 control and 5 myelopathic). Intra-class correlation coefficients (ICC) were computed to evaluate the intra-rater and inter-rater reliability of these measures with an ICC greater 0.7 indicating good to very good agreement.
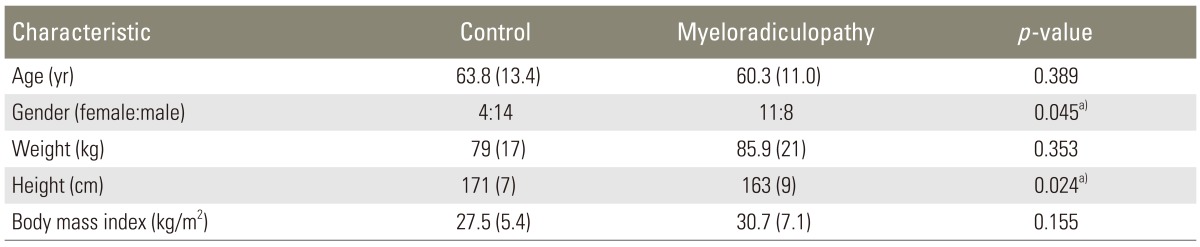
Complete data sets were obtained from 37 identified subjects (18 controls, 19 myelopathic patients) who met all inclusion criteria. Eighteen patients were enrolled in the "control" group with an average age of 60.4±11.0 years (14 male, 4 female). Nineteen patients who met the diagnostic criteria for myelopathy or myeloradiculopathy were enrolled in the "myelopathic" group with an average age of 64.4±13.4 years (8 male, 11 female). The average ages between the two groups did not differ significantly (p=0.32). Demographic data are shown in Table 2.
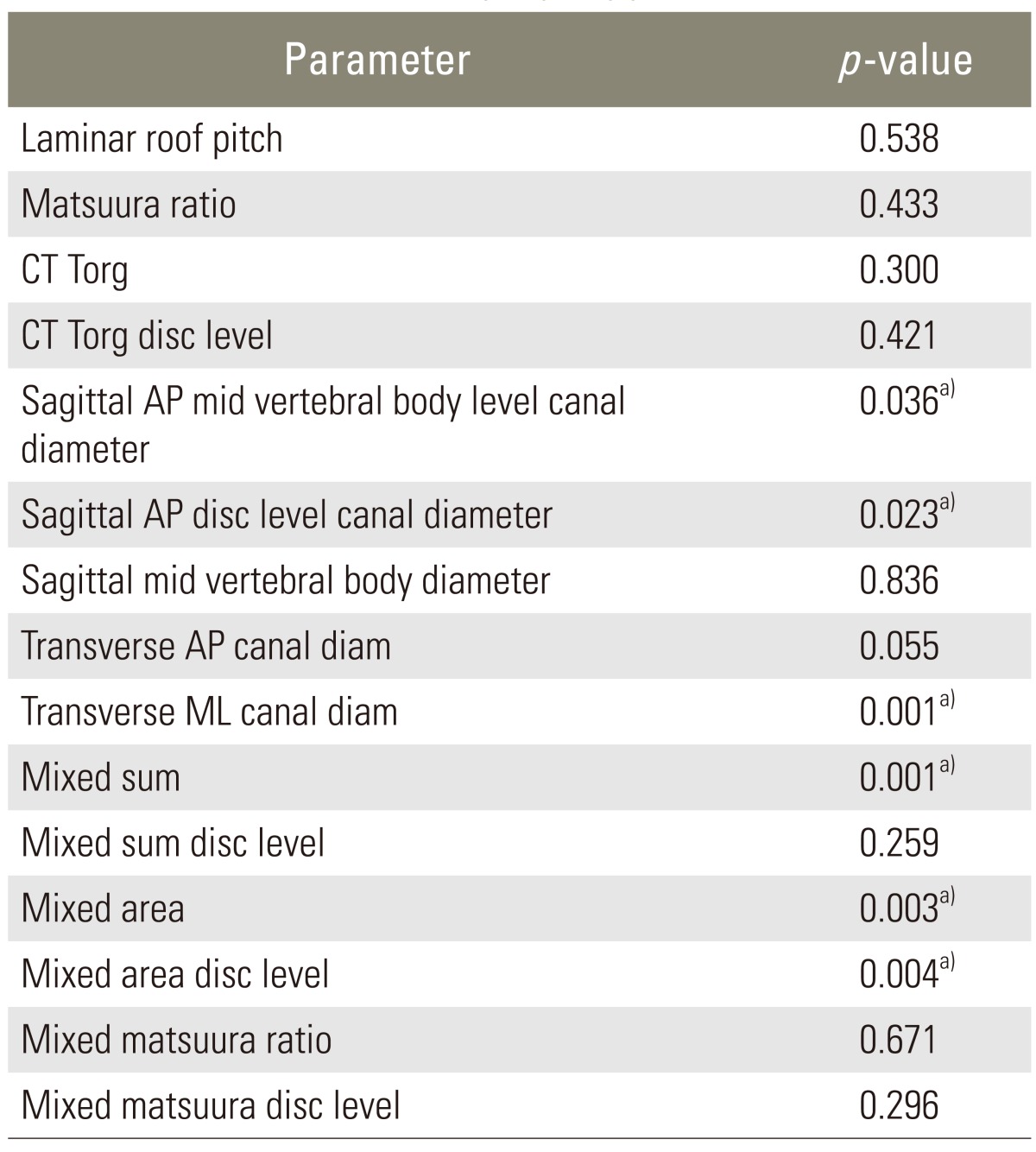
In our study, the two-factor analysis of variance indicated that neither the newly tested parameter LRP (p=0.54) nor the Pavlov-Torg ratio (p=0.30) or Matsuura ratio (p=0.43) differed significantly between control and diseased patients as shown in Table 3. Linear measures including transverse ML canal diameter (p<0.001), sagittal AP canal diameter at midvertebral-height level (p=0.036) and at the disc level (p=0.023) differed significantly between control and myelopathic patients, with the myelopathic group having smaller measured diameters across all cervical levels. Fig. 2 graphically displays the differences in ML measurements between controls and myelopathic patients. Also, mixed sum, a surrogate for canal perimeter (=sagittal AP diameter+transverse ML diameter) and mixed canal area, a surrogate for canal area, (=sagittal AP diameter×transverse ML diameter) were calculated. The term "mixed" emphasizes that calculations were made using parameters measured on sagittal and axial images. Mixed sum (p<0.001) and mixed canal area (p=0.003) were significantly smaller in the myelopathy group.
Sensitivity and specificities of LRP angle, Pavlov-Torg ratio, Matsuura ratio, sagittal AP canal diameter and transverse ML canal diameters were evaluated using receiver operator curves (Fig. 3). At 95% sensitivity, all measures showed a poor specificity ranging from 5% to 16%. The transverse ML canal diameter (followed by the mixed sum of the transverse ML canal diameter and sagittal AP canal diameter) had the largest area under the (receiver operator) curve (area under the curve=0.756) indicating that it was the parameter with the best combined sensitivity and specificity. A transverse ML diameter less that 23.5 mm produced a sensitivity of 82% and specificity of 68%. The mixed sum had a sensitivity of 79% and a specificity of 78% with a 36 mm threshold. The mixed canal area had a sensitivity of 84% and a sensitivity of 67% at 300 mm2 threshold. Both parameters had smaller areas under the curve than transverse ML diameter with the canal area being the smallest. To summarize, no measures were strongly diagnostic, however, a ML diameter less than 23.5 mm had the most accuracy for discriminating between myelopathic and control patients.
The results of the inter-rater and intra-rater reliability assessment confirmed a very good agreement between and within raters for the 4 basic measures (canal diameter, VB, LRP, ML) evaluated in this study. LRP actually had the highest inter-rater ICC (0.928), followed by VB (0.904), ML (0.887) and AP (0.868). The intra-rater reliability between first and second measurements was also very good ranging from 0.859 to 0.973. There was an average of 0.64 mm between the rater measurement error in ML (standard error [SE]+0.07) and of 0.45 within the rater (SE+0.08). This approaches the limits of the precision rating of the Picture Archive and Communications System (Synapse, Fuji Inc., Tokyo, Japan) used for the image measurements.
The transverse ML canal diameter has been proved to have the best receiver operating characteristics with a sensitivity of 82% and specificity of 68% at a threshold less than 23.5 mm. The present study suggests that the ML reserve during a slowly progressive (i.e., atraumatic) cord compression related to cervical spondylosis and disc degeneration is more correlated to a stepwise progressive myelopathy than the Torg-Pavlov ratio, Matsuura ratio or the LRP angle. This may be true because the spinal cord typically responds to ventral compression by flattening in the AP plane and simultaneously expanding in the ML plane. Conversely, we found that while the sagittal AP diameter was significantly higher in controls, it had poor sensitivity and specificity for the differentiation between myelopathic and control patients. This suggests that the mixed sum characteristics are driven by the transverse ML diameter. The mixed area behaved similarly.
Despite the fact that acquired changes are focused at the disc level in cervical spondylotic myelopathy, canal measurements at the disc level do not reliably differentiate between subjects with and without myelopathy. This may indicate that patients developing spondylotic myelopathy have a diffusely narrower canal or it may be simply reflective of the fact that not CT scan evaluated soft tissue changes produce the spinal cord compression responsible for myelopathy.
While LRP did not differ significantly between control and myelopathic patients, it favorably compared with the discriminatory power of Pavlov-Torg ratio, Matsuura ratio and sagittal AP canal diameter. Torg et al. [7] reported a 93% sensitivity and 58% to 59% specificity for a 0.80 threshold for detecting SCI, suggesting that the current data set has more heterogeneity or that the Pavlov-Torg ratio may be a superior discriminator of patients at risk for acute SCI rather than compressive myelopathy from slowly progressive spondylosis and disc degeneration.
In the current study, we noted that the cross-sectional area at the mid-pedicle height level and at the disc level differed between control and myelopathic patients. This contrasts with Matsuura's finding that the area of the canal did not differ between normal patients and those who experienced SCI from a cervical fracture or dislocation [12]. Matsuura retrospectively examined AP and ML dimensions of the cervical spinal canal (C3-C7) in young trauma patients and determined that the ratio of these diameters was predictive of an acute SCI. While the canal cross-sectional areas were equivalent between the control and SCI patients, SCI patients had significantly larger ML dimensions and smaller AP dimensions. The present study suggests that the ML diameter may play more a role in stepwise progressive myelopathy by limiting the expansion of the spinal cord as it attempts to compensate from gradual AP compressive degenerative changes. This is distinctly different from the SCI group in which a restriction in the AP plane correlated with an increased risk of injury. In the SCI population, one mechanism of injury is characterized by a sudden, rapid change in the AP canal diameter, a setting in which the AP reserve, as suggested by Matsuura, is more important than the ML reserve. In studies on SCI, both the Matsuura ratio and Torg-Pavlov ratio have shown their promise in identifying patients at risk for SCI.
These data emphasize that the geometric features of the spinal canal as risk factors for an acute SCI may not be risk factors for a cervical spondylotic myelopathy. While the Matsuura ratio has been used more commonly in the SCI population, the Torg-Pavlov ratio has been previously examined with mixed results in the compressive myelopathy setting. Yue et al. [11] found that patients with spondylotic cervical myelopathy had significantly decreased Torg-Pavlov ratios when compared with non-spondylotic, non-myelopathic patients. Chen et al. [8] reported similar data in Chinese male patients. Suk et al. [10] found that the Torg-Pavlov ratio on plain films correlated with the Torg-Pavlov ratio on CTs and with the vertebral body-to-CSF column ratio in myelopathy patients, but they did not evaluate these measures in asymptomatic controls. Lim and Wong [9] revealed that the vertebral body sagittal diameter variability led to Torg-Pavlov ratio variations across different ethnicities and between genders within the same population. In their study, the Torg-Pavlov ratio did not consistently distinguish patients with cervical stenosis. Collectively, these data suggest the Torg-Pavlov ratio may be most helpful in identifying myelopathy patients within an ethnically uniform population. The current data set was not controlled for race and ethnicity and these factors may represent sources of variability, which compromised the discrimination of the Torg-Pavlov ratio [7,14].
The current study has several limitations, which must be addressed in subsequent follow-up studies. The significant differences in gender and height between the cohorts are two anthropomorphic factors that were not controlled in this comparative study, but may impact the results. The potential for systemic bias exists because control and myelopathic patients were scanned using a different equipment. Since a power analysis was not performed for this study, the relatively small cohort sizes may have induced type II error, since several of the parameters failed to show significant differences between cohorts and/or displayed poor diagnostic characteristics based on the receiver operator curve analysis. Future confirmation studies should use the data reported herein to perform a priori power analysis and involve properly sized cohorts with a more controlled enrollment scheme to minimize the effects of such bias. In addition, cases with OPLL were excluded from our study as well as this condition alters the measured dimensions of the bony spinal canal. Thus, this study does not refer to the subset of patients who develop cervical myelopathy in the setting of OPLL.
The identification of reliable and accurate measures of spinal canal dimensions that correlate with cervical myelopathy could help in the diagnosis and treatment recommendations for these patients. Our preliminary finding of the importance of the ML dimension of the canal is encouraging, as it is a parameter that has been previously considered important in the development of cervical myelopathy. The current study would suggest that an absolute restriction (<23.5 mm) in the transverse ML diameter may identify a subset of patients at increased risk of compressive myelopathy irrespective of other measures of spinal canal parameters. This may represent a form of congenital stenosis similar to the short pedicle syndrome seen in the lumbar spine. Unfortunately, this study showed that previously reported ratios measures and our own new one (LRP) fail to reliably discriminate between patients who do and do not develop compressive cervical myelopathy. The results of this study should be expanded upon in further work on this topic.
Acknowledgments
We would like to specifically acknowledge Ms. Martha Mars (Landstuhl Regional Medical Center) for her diligent support of this work.
References
1. Veidlinger OF, Colwill JC, Smyth HS, Turner D. Cervical myelopathy and its relationship to cervical stenosis. Spine (Phila Pa 1976) 1981 6:550–552. PMID: 7336277.


2. Turnbull IM. Microvasculature of the human spinal cord. J Neurosurg 1971 35:141–147. PMID: 5570776.


3. Payne EE, Spillane JD. The cervical spine; an anatomico-pathological study of 70 specimens (using a special technique) with particular reference to the problem of cervical spondylosis. Brain 1957 80:571–596. PMID: 13499761.



4. Hinck VC, Gordy PD, Storino HE. Developmental stenosis of the cervical spinal canal. Radiological considerations. Neurology 1964 14:864–868. PMID: 14215604.


5. Prasad SS, O'Malley M, Caplan M, Shackleford IM, Pydisetty RK. MRI measurements of the cervical spine and their correlation to Pavlov's ratio. Spine (Phila Pa 1976) 2003 28:1263–1268. PMID: 12811269.


6. Shedid D, Benzel EC. Cervical spondylosis anatomy: pathophysiology and biomechanics. Neurosurgery 2007 60:S7–S13. PMID: 17204889.



7. Torg JS, Naranja RJ Jr, Pavlov H, Galinat BJ, Warren R, Stine RA. The relationship of developmental narrowing of the cervical spinal canal to reversible and irreversible injury of the cervical spinal cord in football players. J Bone Joint Surg Am 1996 78:1308–1314. PMID: 8816644.


8. Chen IH, Liao KK, Shen WY. Measurement of cervical canal sagittal diameter in Chinese males with cervical spondylotic myelopathy. Zhonghua Yi Xue Za Zhi (Taipei) 1994 54:105–110. PMID: 7954043.

9. Lim JK, Wong HK. Variation of the cervical spinal Torg ratio with gender and ethnicity. Spine J 2004 4:396–401. PMID: 15246298.


10. Suk KS, Kim KT, Lee JH, Lee SH, Kim JS, Kim JY. Reevaluation of the Pavlov ratio in patients with cervical myelopathy. Clin Orthop Surg 2009 1:6–10. PMID: 19884991.



11. Yue WM, Tan SB, Tan MH, Koh DC, Tan CT. The Torg-Pavlov ratio in cervical spondylotic myelopathy: a comparative study between patients with cervical spondylotic myelopathy and a nonspondylotic, nonmyelopathic population. Spine (Phila Pa 1976) 2001 26:1760–1764. PMID: 11493847.


12. Matsuura P, Waters RL, Adkins RH, Rothman S, Gurbani N, Sie I. Comparison of computerized tomography parameters of the cervical spine in normal control subjects and spinal cord-injured patients. J Bone Joint Surg Am 1989 71:183–188. PMID: 2918002.


13. Rhee JM, Riew KD,Cervical spondylotic myelopathy: including ossification of the posterior longitudinal ligament. Spivak JM, Connolly PJ,North American Spine Society. editors. Orthopaedic knowledge update. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2006. p.235–248.
14. Pavlov H, Torg JS, Robie B, Jahre C. Cervical spinal stenosis: determination with vertebral body ratio method. Radiology 1987 164:771–775. PMID: 3615879.


15. Devore JL. Probability and statistics for engineering and the sciences. Belmont: Duxbury Press; 1991.
16. Herzog RJ, Wiens JJ, Dillingham MF, Sontag MJ. Normal cervical spine morphometry and cervical spinal stenosis in asymptomatic professional football players. Plain film radiography, multiplanar computed tomography, and magnetic resonance imaging. Spine (Phila Pa 1976) 1991 16:S178–S186. PMID: 1862411.


Fig. 1
(A) Sagittal image measurements included the anterior-posterior (AP) canal diameter measured at mid-vertebral body level (CD) and the disc level (CDDL) and the diameter of the vertebral body (VB). (B) Axial image measurements included the laminar roof pitch (LRP) angle, the AP diameter of the canal and the medial-lateral (ML) diameter of the canal.
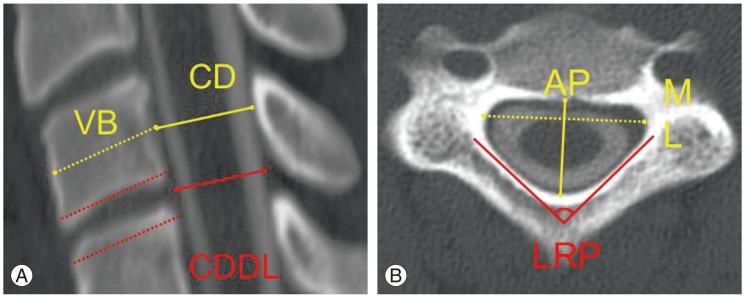
Fig. 2
Comparison of control vs. myelopathic patients using transverse medial-lateral (ML) canal diameter from C2-C7. Error bars indicate standard deviation.
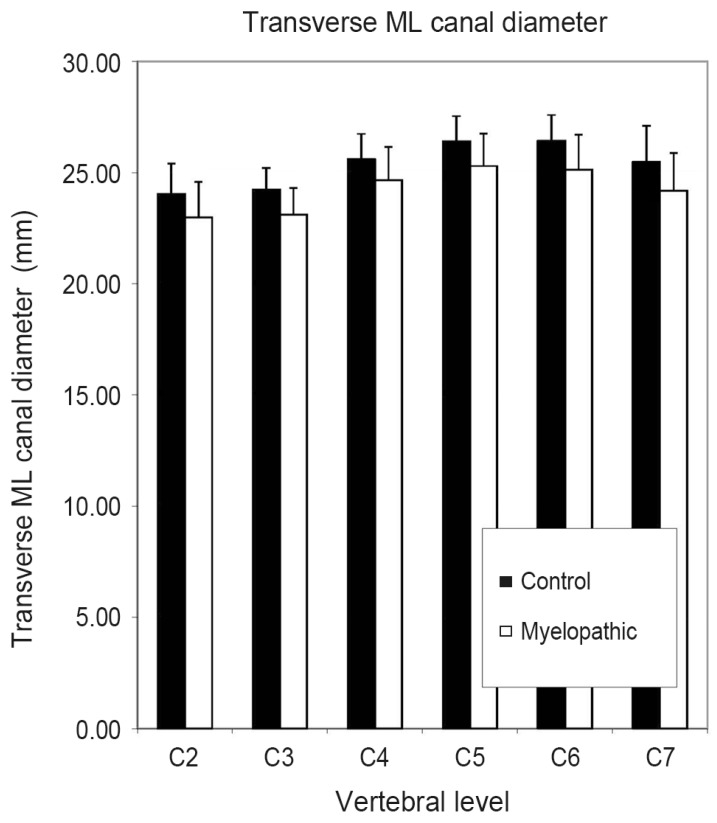
Fig. 3
Receiver operator curves for Pavlov-Torg ratio, Matsuura ratio, mixed sum, mixed area, sagittal AP canal diameter and transverse ML canal diameters. The transverse ML diameter threshold less than 23.5 mm is indicated by an asterix (*). AP, anterior-posterior; ML, medial-lateral.
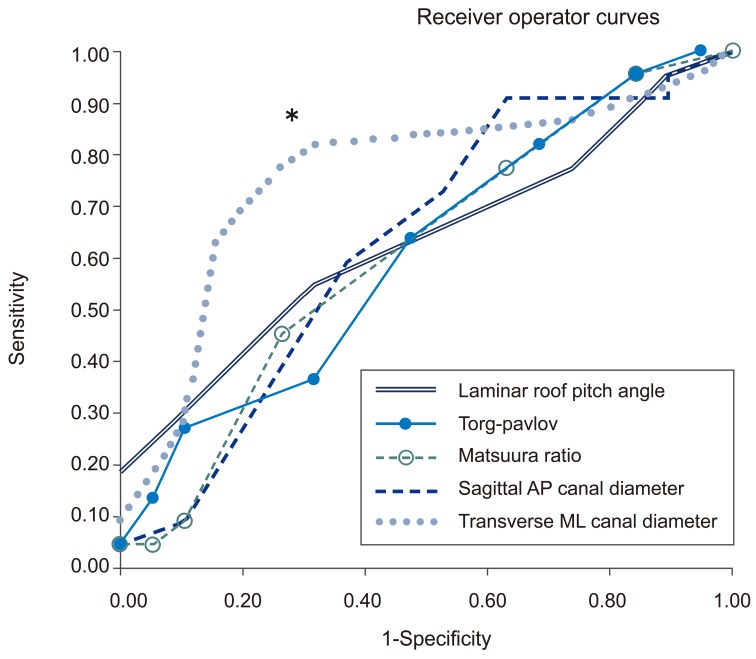
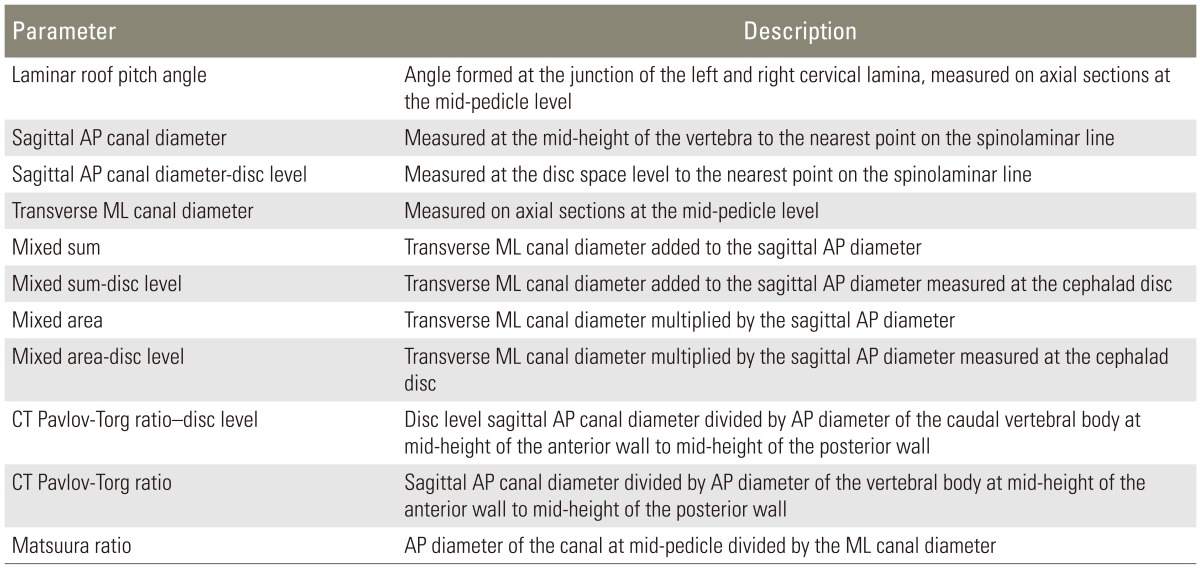
Table 1
Definitions of geometric parameters measured and calculated from axial and sagittal computed tomography images of the cervical spine
- TOOLS