Adjacent Segment Pathology after Lumbar Spinal Fusion
Article information
Abstract
One of the major clinical issues encountered after lumbar spinal fusion is the development of adjacent segment pathology (ASP) caused by increased mechanical stress at adjacent segments, and resulting in various radiographic changes and clinical symptoms. This condition may require surgical intervention. The incidence of ASP varies with both the definition and methodology adopted in individual studies; various risk factors for this condition have been identified, although a significant controversy still exists regarding their significance. Motion-preserving devices have been developed, and some studies have shown their efficacy of preventing ASP. Surgeons should be aware of the risk factors of ASP when planning a surgery, and accordingly counsel their patients preoperatively.
Introduction
Spinal fusion stops not only the progression of spinal pathology, but it also immobilizes the painful motion segment. Further, it also stabilizes the spine after neural decompression. Hence, spinal fusion has been used increasingly in the treatment of various lumbar diseases. Moreover, this trend is being further accelerated by the development of surgical techniques and spinal instrumentation. One study reported that the annual number of lumbar spinal fusion performed in the United States has rapidly increased by 2.7 times during the past decade [1]. However, the increase of mechanical stress and segmental motion at adjacent segments after spinal fusion has been reported [234] and as a consequence, various pathologies of adjacent segments, including acceleration of degenerative changes, have been reported (Fig. 1). It is assumed that these pathologies occur under the direct or indirect influence of biomechanical changes at adjacent segment to spinal fusion. Symptomatic degenerative changes in the adjacent segments affect a patient's functional outcome, and may also require surgery [5678]. Therefore, it is believed that a spine surgeon who considers a patient's long-term outcome should understand the characteristics of adjacent segment pathology (ASP) more accurately, and be more careful in order to reduce the incidence of these pathologies. This review aims to review the definition, incidence and risk factors of ASP, and the effect of motion-preserving surgeries on its incidence of development.
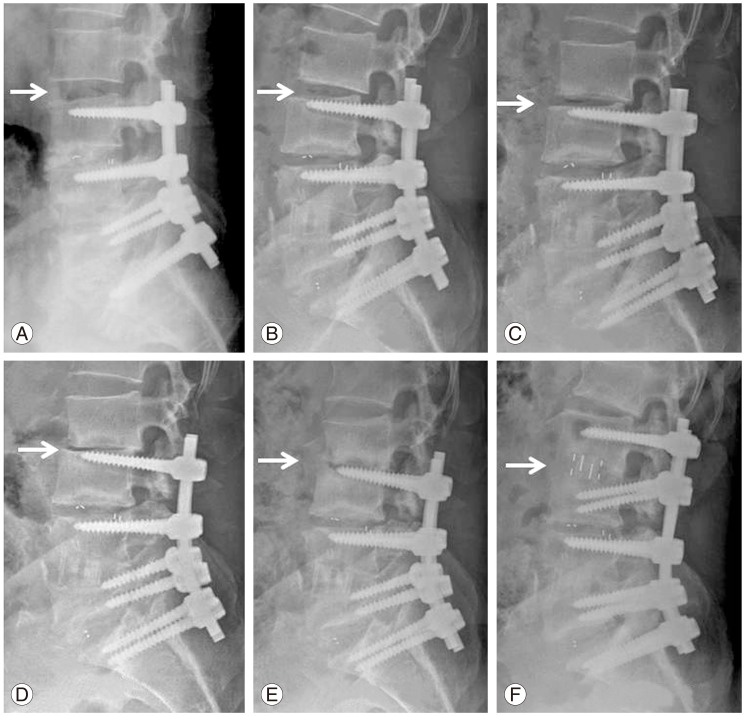
Progressive degeneration of proximal adjacent segment (arrow indicates the level of adjacent segment pathology) was observed after lumbar spinal fusion from L3 to S1 in 57-year-old gentleman. Progressive settling, development of retrolisthesis, and segmental kyphosis was gradually developed in the adjacent segment during follow-up. The patient was treated by minimally invasive direct lateral interbody fusion of the adjacent segment and posterior extension of fusion. Postoperative (A) 3 months, (B) 6 months, (C) 9 months, (D) 12 months, (E) 18 months, and (F) after revision.
Definition
Hilibrand and Robbins [9] classified ASPs that developed subsequent to spinal fusion, into two categories, describing them as "adjacent segment degeneration" and "adjacent segment disease" [9]. They described the radiographic changes of adjacent segment to spinal fusion as "adjacent segment degeneration" and defined that it is not necessarily correlated with clinical symptoms. On the other hand, they defined "adjacent segment disease" as clinical symptoms correlated with radiographic changes adjacent segment to previous fusion. Park et al. [8] have asserted that adjacent segment diseases include any abnormal process in mobile segments adjacent to spinal fusion; although disc degeneration was the most commonly observed among those abnormal processes, it should also include listhesis, instability, facet joint degeneration, herniated disc, stenosis, scoliosis, compression fracture, etc. More recently, Riew et al. [10] proposed the term "adjacent segment pathology", which is relatively simple and includes any change that occurs adjacent to a previously operated level; this is because the term "adjacent segment degeneration or disease" is ambiguous, and is unable to include multifactorial processes. Furthermore, they subdivided ASP into "radiographic ASP" (RASP) and "clinical ASP" (CASP); the RASP means those change which occur at adjacent segments and are detected only on radiography, whereas the CASP means pathologies accompanied with clinical signs and symptoms. Moreover, it has yet to be defined as up to what segment should be included in the "adjacent segments". Generally, adjacent segment is thought to be the immediate next segment to the spinal fusion. Some articles insist that it is within two segments adjacent to the spinal fusion [7111213]. There are some who have reported that degeneration is more frequently found at the first adjacent segment to fusion than at the second adjacent segment [1415]. However, other reports state that reduction of the second adjacent segment disc height was observed at a similar rate with that of the first adjacent segment [71617]. Thus, it is unclear and difficult to determine the limits for adjacent segment influenced by fusion. The authors believes that it is reasonable to define ASP up to the second adjacent segment to fusion, and recommend future researchers to analyze data by classifying ASP at the first and ASP at the second adjacent segment, if the number of patients is enough.
Incidence
In a review of 22 existing studies, incidence estimates in the literature of ASP varies widely from 5.2% to 100%; this wide range of estimate could be due to the different definitions, methodologies, and follow-up period in each study [8]. The annual incidence of ASP is relatively low, and requires substantial work to understand. Thus, many studies are based on radiographic findings, which are quicker to conduct and generally lead to a higher incidence rate; however, their diagnostic criteria do not have a robust correlation with clinical symptoms. In the review of 27 studies by Harrop et al. [18], the incidence of RASP ranged from 8% to 100% but CASP ranged from 0% to 27.5%. This result suggests that radiographic degenerative changes at adjacent segments are common; however, clinical symptoms are less likely to be manifested. Another review by Park et al. [8] found the overall incidence of 'radiographic' ASP was between 8% and 100%; this finding is substantially higher than the 5.2% to 18.5% of range which is the typically reported incidence of 'symptomatic or clinical' ASP in literature. Furthermore, the incidence rate of revision surgery for ASP has an even lower report rate in literature, being 2% to 15% only. These data may suggest that RASP is common after lumbar spinal fusion; however, adjacent segment lesions, which are symptomatic or clinically significant enough to require a revision surgery, are relatively rare [11].
In studies requiring prolonged observation time, such as ASP, survival analysis is a useful method, since a proportion of patients are inevitably lost to follow up. All studies last for a finite time span, and it is difficult to know when the remaining patients will experience the ASP. Survival analysis allows one to calculate the incidence of ASP requiring surgery at the given time, and to predict prevalence of the disease over the long term. In recent studies, researchers have increasingly used survival analysis because one can calculate and statistically predict true incidence and prevalence of ASP in the total number of patients, including patients lost to follow-up.
In three studies (which will be introduced in the following text), the prevalence of ASP was predicted using the survival analysis. In the long-term follow-up retrospective study by Ghiselli et al. [11], 215 patients were followed up for an average of 6.7 years, and the predicted rates of patients needing additional surgeries for adjacent segment diseases were 16.5% at 5 years, and 36.1% at 10 years. Sears et al. [19] enrolled 1,000 patients in a consecutive series, having an average 63 months follow-up study. As a result of the study, they reported that in a 10-year survival analysis, the prevalence of further surgery in the adjacent segment was 22.2%. Moreover, reoperation rate is thought to be influenced by socioeconomic or cultural factors. In a study performed in Asia, Lee et al. [12] reported that based on the results of survival analysis of 490 patients treated with lumbar fusion, the annual incidence of ASP requiring surgery was 1.2%, and 10% of the patients needed reoperation within 10 years. This revision surgery rate is lower than the results of United States and Australian articles mentioned above. It may reflect that Asians tend to be reluctant to undergo surgeries and, in particular, reoperation.
Biomechanical Studies
Various studies have been conducted under the hypothesis that biomechanical changes at the adjacent segment after lumbar spinal fusion results in ASP. These studies are largely divided into two categories: studies that investigated the increased stress on the adjacent segment, and others that did the increased motion on it.
In the early landmark report that identified the increased stress on the adjacent segment, Lee and Langrana [2] carried out their study on 16 cadavers and reported that the stress increased on the adjacent segment to the fusion, and there was an increased loading on the facet joint of the adjacent segment. In another cadaveric study, Weinhoffer et al. [20] observed that intradiscal pressure of adjacent segments increased during flexion. They also found that when more segments were fused, the increase in pressure was greater.
Increased motion at the adjacent segments was also suggested as a potential cause of ASP after lumbar spinal fusion. In a study conducted with canine spines, Ha et al. [21] found increased segmental mobility and changed contact pattern of the facet joints in the adjacent segment after spine instrumentation. In a radiographic analysis, Axelsson et al. [22] found increased motion of adjacent segments after L4-5 spinal fusion.
Risk Factors
Symptomatic degenerative changes in the adjacent segments affect the patient's functional outcome, and may ultimately require surgery [5678]. Accordingly, many studies have attempted to identify risk factors to reduce the incidence of these ASPs. However, this topic has been highly controversial. We attempt to review by categorizing the pre-existing variables before the spinal fusion, and surgery-related variables determined by a surgeon's choice during surgery (Table 1).
Pre-existing Variables
1. Age
The factor which has been commonly pointed out by many studies as an ASP risk factor (one of the most important risk factors) is age at the time of the primary surgery [1217192324252627]. It is thought to be attributable to the decreased ability of the spine to accommodate the biomechanical changes induced by a fusion in old age, or to an ongoing disc degeneration in old age. Aota et al. [23] found the incidence of segment instability adjacent to the spinal fusion was significantly higher in patients older than 55 years of age. Lee et al. [12] observed survivorship of the adjacent segment after lumbar spinal fusion: the authors found that only 78% of adjacent segments survived 10 years postoperative in patients older than 60 years of age, which is significantly lower than a 93% survival rate in patients aged less than 60 years. However, some studies have reported no correlation between ASD incidence and the patient's age [11282930].
2. Adjacent segment disc degeneration
If there is already a degenerated disc in adjacent segment to the spinal fusion, the degeneration of this segment may progress faster. Edwards et al. [31] followed up 34 patients with fusion extending from the thoracic spine to L5 for an average of 5.6 years, and found that L5-S1 disc degeneration occurred in 61% of patients. In addition, they reported that the presence of even mild radiographic degeneration at L5-S1 disc was one of risk factors. However, there has been very few studies that identify whether a pre-existing disc degeneration can be a predisposed risk factor for ASP occurrence or not. Interestingly, contrary to this, many studies have reported that the presence of disc degeneration at the adjacent segment did not induce to increase the ASP incidence [11303233]. For example, in the study on the revision surgery rate of adjacent segment in 215 lumbar fusion patients, Ghiselli et al. [11] reported that pre-existing degeneration at the adjacent segment had no correlation with the ASP incidence.
3. Facet degeneration or tropism of adjacent segment
Pre-existing facet degeneration before lumbar fusion has been suggested as a risk factor of ASP incidence. Lee et al. [30] reported that revision surgery for ASP was needed in 2.62% of 1,069 patients who underwent fusion, and pre-existing facet degeneration was the only significant risk factor involved. Okuda et al. [33] also reported that the incidence of ASP was high when there was tropism on an adjacent level facet. On the other hand, there is also opposite contradicting report that facet tropism at the adjacent segment was not related to ASP [32]. Therefore, we believe that further studies are required to identify the effect of the facet conditions of adjacent segments on ASP.
Surgery-Related Variables
1. Number of fusion segments
Previous studies have generally reported increasing incidence of ASP when number of fused segments increase by the longer lever arm and stress transfer phenomenon [17192324262836]; however, other studies could not detect a significant difference [111215252937]. Sears et al. [19] found that the fusion of 3 or 4 levels increased the risk of revision surgery by three times when compared to single-level fusions. However, Ghiselli et al. [11] performed survival analysis on a cohort of 215 patients who were treated by lumbar spinal fusion for lumbar degenerative disease. They found that the revision surgery rate for adjacent segment degeneration was three times higher in single level fusion patients than multiple-level. They postulated that the incidence of adjacent segment disease decreased in multi-level fusion patients, because the number of remaining segments decreases as the number of fused segments increases. In a survival analysis of 490 patients treated by 1-3 segment fusion without significant spinal deformity, authors could not find a statistical significance for single-level fusion versus fusion of 2 or 3 levels [12]. Even though authors should be careful before coming to a hasty conclusion, they suggested that longer segment fusion does not necessarily result in a higher revision rate for ASP than shorter segments fusion.
2. Adjacent segment damage during surgery
Disruption of posterior elements changes the normal anatomy and biomechanics, and potentially predispose to ASP. Lai et al. [38] found that sacrifice of the posterior elements from spinal fusion to adjacent segments increased the risk of adjacent segment instability up to 3 times. The upper adjacent facet joint injury which may occur during insertion of a pedicle screw is also known as a cause to increase the risk of ASP [152324].
On the other hand, there have been reports that in patients treated by anterior fusion, this posterior structure was not damaged, and the ASP incidence was significantly low [3940]. This issue will be addressed in detail in the following section, where we will discuss the differences in the ASP incidence according to fusion methods.
One researcher has reported that decompressive surgery on the segment adjacent to the fusion causes an increase of ASP incidence. Sears et al. [19] found performing a laminectomy on the adjacent level to the spinal fusion increases the rate of revision surgery on the adjacent segment by 2.4 times. In contrast, Aiki et al. [28] reported that adjacent level decompression does not increase the second surgery frequency after lumbar spinal fusion. Lee et al. [12] also could not find any significant difference in frequency of secondary surgery on the adjacent segments, irrespective of whether decompression was performed or not. They believe that if a surgeon uses a meticulous technique and preserves supporting structures as much as possible when neural decompression of adjacent segment is performed, laminectomy on adjacent segment may not adversely increase incidence of secondary surgery for ASP.
3. Fusion methods
Overall, most of the studies reported that there are no significant differences in ASP incidence between fusion methods. Abdu et al. [41] reported that there were no differences in clinical outcome and additional surgery rate between three groups of degenerative spondylolisthesis patients treated with posterolateral in situ fusion, posterolateral fusion (PLF) with pedicle screws, or 360° fusion with pedicle screws. In 83 consecutive patient series, Kumar et al. [29] could not find any significant differences in the incidence of adjacent segment degeneration between the PLF group and another group that underwent PLF supplemented with additional posterior lumbar interbody fusion (PLIF). Cheh et al. [42] also reported there was no difference in incidence of RASP between the posterior fusion group and the circumferential fusion group in 188 patients, having a minimum 5-year follow-up. Videbaek et al. [43] also reported that the ASP incidence was not different between the PLF group and the group treated with 360° fusion achieved by PLF combined with anterior lumbar interbody fusion.
However, a few studies have reported a difference in the incidence of ASP based on different fusion methods; an interbody fusion particularly increased the risk. Rahm and Hall [26] found that addition of PLIF to PLF increased adjacent segment degeneration. Lee et al. [12] reported significantly higher incidence of ASP in patients treated by PLIF than PLF, in the long-term follow-up of 490 patients.
Several studies reported that the patients treated with anterior interbody fusion showed very low ASP incidence because their posterior structures were not damaged (Fig. 2). Wai et al. [40] did a follow-up for 20 years after non-instrumented anterior lumbar interbody fusion, and magnetic resonance imaging studies revealed ASP incidence. As a result, only 6% of patients underwent surgery for ASP, and there were no differences in the ASP incidence when compared to other non-adjacent segments. Min et al. [39] reported the RASP incidence in spondylolisthesis patients. They found 44% of patients developed RASP after anterior interbody fusion, and it was significantly lower than 83% of incidence after posterior interbody fusion.
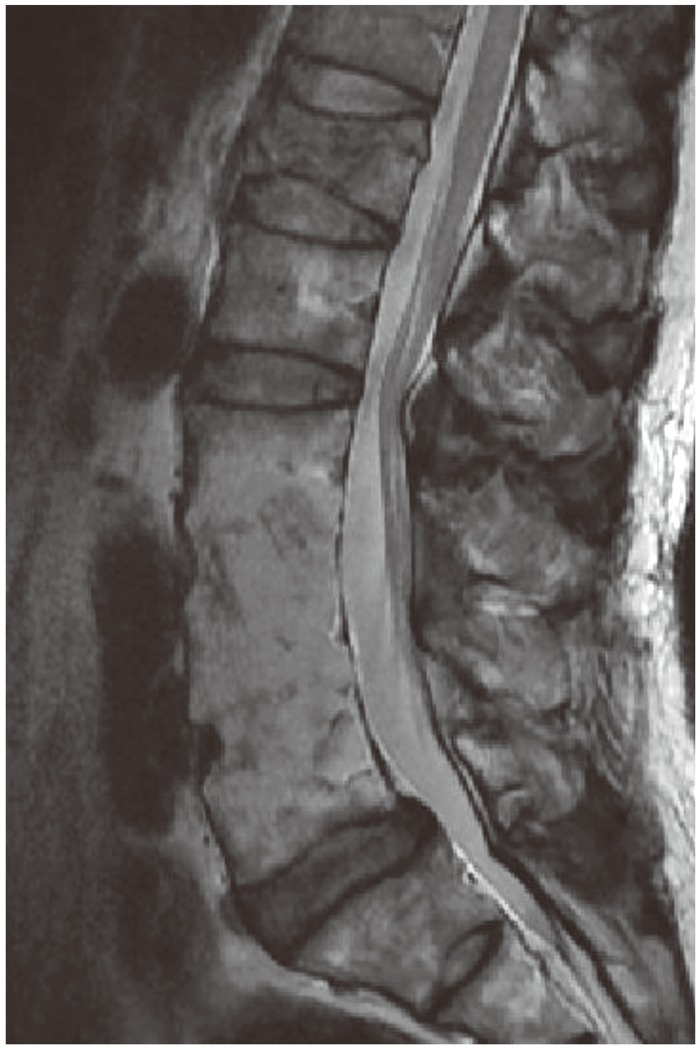
A 65-year-old gentleman visited our clinic due to recent compression fracture of L1. He underwent anterior fusion of L3-4 and L4-5 at another hospital for pyogenic infection. Interestingly, magnetic resonance imaging reveals minimal degeneration of adjacent segments at 22 years after anterior fusion. His condition was well without the back pain before this compression fracture.
Besides, there have been reports that the increased rigidity due to instrumentation led to an increase of ASP incidence [132324]. However, many studies also reported that the addition of instrumentation did not cause an increase in the ASP [1115282944]. Nowadays, for spinal fusion is rarely performed without instrumentation, since this methodology increases the fusion rate. Thus, the clinical significance for this issue has decreased.
4. Sagittal alignment
Abnormal sagittal alignment after spinal fusion is thought to be a cause of biomechanical alteration and ASP [2829]. It has been proposed that various parameters of spinal alignment may affect the ASP incidence. Kawakami et al. [45] asserted that both preoperative L1 axis S1 distance and lordosis at follow-up, are important for clinical outcome after PLF for degenerative spondylolisthesis patients. Kumar et al. [29] reported that the ASP incidence was reduced by restoring the normal C7 plumb line and sacral inclination on immediate postoperative radiographs.
Moreover, there have been reports that alignment of fusion in lumbar spine affected the incidence of ASP. Soh et al. [46] reported that restoring the segmental angle in lumbar fusion to more than 15° has a protective effect on ASP.
5. Floating fusion
Traditionally, many surgeons believed that L5-S1 should be routinely included in the fusion for pathology at L4-5. In comparison, the fusion involving only L4-5 was called floating fusion. However, there has been paucity of literature which supports this belief. In a study comparing L4-5, L5-S1 and L4-5-S1 fusion, Disch et al. [47] reported that L4-5 fusion caused ASP more frequently than L5-S1 or L4-5-S1 fusion, and L4-5 floating fusion is a risk factor of ASP.
In case of short fusion in the lower lumbar spine, floating fusion may be well tolerated. Traditionally, there have been concerns that accelerated degeneration may occur at L5-S1 after L4-5 fusion, and this may lead to an increase in the incidence of revision surgery. However, contrary to these concerns, many studies reported that L5-S1 is not required to be routinely included in fusion. In the comparative study with a minimum of 5-year follow-up of 107 patients with L4-5 degenerative spondylolisthesis accompanied with L5-S1 disc degeneration, Liao et al. [48] reported that there were no differences in clinical results between the floating fusion (L4-5 fusion only) group and non-floating fusion group (the L4-L5-S1 lumbosacral fusion). The incidence of RASP at L5-S1 was higher in floating fusion; however, it was mostly asymptomatic. On the other hand, proximal ASP occurred more frequently in the lumbosacral fusion group. They concluded that the routine extension of fusion to sacrum could not reduce the incidence of revision surgery. In a follow-up study of more than 5 years, of 54 patients treated with PLIF for their spondylolisthesis at L4-5, Miyakoshi et al. [14] also reported that there were no differences in clinical results when comparing groups with and without preoperative disc space narrowing at L5-S1. Hence, L5-S1 segment does not need to be involved in fusion although there is disc degeneration. Ghiselli et al. [49] also reported an average 7.3-year follow-up of L4-5 posterior spinal fusion. They found that there was neither increased symptomatic disc degeneration nor symptoms necessitating L5-S1 fusion after follow-up.
In case of thoracolumbar long segments fusion, there has been an ongoing controversy about which method is better: floating fusion by stopping the fusion at L5, or lumbosacral fusion by extending up to the sacrum. It is generally believed that L5-S1 should be involved in long fusion. Edwards et al. [31] reported a mean follow-up of 5.6 years of 34 patients, who had fusion from the thoracic spine to L5. They found that 61% of patients subsequently developed L5-S1 advanced disc degeneration, and needed revision surgery. Sears et al. [19] also reported that stopping fusion at L5 leads to a greater risk of symptomatic ASP, when compared with fusion to sacrum.
Effect of Motion-Preserving Technologies on ASP
1. Dynamic stabilization
Surgeons supporting the motion preserving or dynamic stabilization methods believe that preserving the motion of index segment has advantages; degenerative change of adjacent segment is not accelerated with this method, and risk of ASP can also be reduced potentially. For supporting this view, there have been many studies that compared the incidence of ASP based on the use of fusion method and dynamic stabilization. Various devices, including hinged pedicle screw [50], ligamentoplasty [51], and nitinol spring rod system [52] were used in these studies. Recently, in a meta analysis, Ren et al. [53] reported that it was effective because radiographic or symptomatic ASP and reoperation rate were significantly lower in the dynamic posterior instrumentation group, compared to the fusion group. On the other hand, some researchers reported that there were no differences in adjacent level range of motion [54] and adjacent disc degeneration [55] when comparing dynamic stabilization and fusion. Schmoelz et al. [5657] casted doubts on the effect of dynamic stabilization in their biomechanical study with cadavers, because this method could preserve only motion of the index segment, and the intersegmental motion of the adjacent segment was not significantly different; also, the intradiscal pressure of the adjacent segment was similar compared to the rigid fixator. In a recent systemic review, posterior motion preserving and dynamic stabilization techniques showed low to insufficient evidence that these techniques are superior to fusion in preventing ASP. In conclusion, motion preserving techniques when compared to classic fusion surgery, preserve the motion of index level for some time and it tends to decrease the ASP incidence. However, the evidence is insufficient and there has been no well-designed study involving a long-term follow-up.
Total Disc Replacement
There is a relatively long history in the development of total disc replacement (TDR). This technique received attention again since the mid-1990s because of ASP, a long-term complication of fusion surgery. In theory, it has both advantages of motion preservation at the index level, and the posterior ligament complex preservation of the adjacent segment. Therefore, it was expected to show excellent effect for prevention of ASP. Relatively long-term follow-up studies on TDR have been reported in patients who received the CHARITÉ artificial disc implant. Putzier [58] reported 53 patients that underwent a CHARITÉ disc arthroplasty procedure with a 17-year follow-up. The authors noted a 17% incidence of RASP. David [59] reported on single level (either L4-L5 or L5-S1) CHARITÉ arthroplasty in 106 patients with an average 13.2-years follow-up. The authors reported a 2.8% incidence of CASP at follow-up. In the review on the incidence of radiographic or CASP, Harrop et al. [18] reported that 34% in the fusion group and 9% in the arthroplasty group developed RASP, and the development of CASP was 14% in the fusion group and 1% in the arthroplasty group. Both radiographic and CASP has a reduced occurrence after arthroplasty when compared to fusion, and the differences were statistically significant. In a recent systemic review, Wang et al. [60] reported that ASP was six times less likely to occur in the TDR or motion preserving surgery group as compared to the fusion group; this result indicated the 'moderate' evidence. In conclusion, TDR reduces the incidence of ASP, as compared to classic fusion surgery. However, the evidence is not enough since long-term follow-up data is not yet available. Accordingly, further studies are needed.
Conclusions
ASP may develop after lumbar spinal fusion, and may negatively affect the patients' clinical outcome. Furthermore, a proportion of patients may require further revision surgery. Radiographic changes of adjacent segment are common after lumbar spinal fusion; however, CASP is less common, and hence the rate of revision surgery for ASP is less. Many risk factors for ASP development have been identified in numerous studies, even though they are not always consistent in all the studies. Spine surgeons should be aware of potential risk factors for development of ASP. These factors should be included in the initial surgical planning and patients' counseling.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
