Autologous Conditioned Serum as a Novel Alternative Option in the Treatment of Unilateral Lumbar Radiculopathy: A Prospective Study
Article information
Abstract
Study Design
The study was conducted on patients who received autologous conditioned serum (ACS) as a line of treatment at the Orthopedics outpatient department of Post Graduate Institute of Medical Education and Research (PGIMER, Chandigarh) from January 2011 to June 2012. Of the 1,224 patients, 20 males or females were included in the study based on the inclusion and exclusion criteria. The institutional board of PGIMER approved the study before it was initiated.
Purpose
To study the efficacy of ACS in the treatment of unilateral lumbar radiculopathy.
Overview of Literature
Interleukin (IL)-1 appears to be of special importance among the cytokines identified in orthopedic diseases. ACS contains high concentrations of IL-1 receptor antagonist, antagonist to IL-1 in that is a biochemical 'sensitizer' of nerve roots in radiculopathy.
Methods
We included 20 patients with unilateral lumbar radiculopathy after obtaining informed consent. We prepared ACS as described by Meijer et al. Under bi-planar fluoroscopic imaging in anterior-posterior and lateral views, ACS was administered via epidural perineural technique. Patients in both groups were evaluated by quadruple visual analogue scale, straight leg raising test, revised Oswestry disability index, and 12-Item Short Form of Health Survey before and after epidural injections at 3 weeks, 3 months, and 6 months.
Results
There was a statistically significant change in all parameters from pre-injection to first, second, and third follow-up (p<0.001).
Conclusions
ACS can modify the disease course in addition to reducing pain, disability and improving general health.
Introduction
Low back pain (LBP) is the most common musculoskeletal disorder of industrialized society, and the most common cause of disability in persons younger than 45 years of age [1]. Disc degeneration is one of the main reasons for chronic LBP, accounting for 39% incidence [2]. Disc herniation-associated radiculopathy is both a biochemical and a mechanical disorder. Nucleus pulposus contains a variety of inflammatory pain mediators, including phospholipase A2, nitric oxide and prostaglandin E [3].
Cytokines such as interleukin (IL)-1 have been identified as pivotal mediators of inflammatory and degenerative changes affecting components of the musculoskeletal system, including the lumbar spine. The presence of disc material in the epidural space initially results in direct toxic injury to the nerve root by chemical mediation, and subsequently, amplifies the intra- and extra-neural swelling that results in venous congestion and conduction block [4]. However, neither the size nor the location of the herniated disc correlates with pain [5].
Of the cytokines identified in orthopedic diseases, IL-1 appears to be of special importance [6]. The therapeutic use of IL-1 inhibitors in such diseases was proposed and has formed the basis for the development of new biological treatment modalities. Strategies for inhibiting the biological activities of IL-1 include the use of IL-1 receptor antagonist (Ra), soluble forms of IL-1 receptors, and type 1 cytokines such as IL-4, IL-10, and IL-13 that inhibit the synthesis of IL-1, increase the synthesis of IL-1Ra or both. The therapeutic use of cytokine inhibitors and growth factors was first proposed in the late 1970s and early 1980s. Growth factors, which target the revitalization of cytodamaged tissue like prolapsed disc function by modulating the underlying pathologies have an increasing role in the local treatment of lumbar radiculopathy [1].
Autologous conditioned serum (ACS) preparations are a source of anti-inflammatory cytokines, including IL-4, IL-10, IL-13 and IL-1Ra and also contain elevated concentrations of growth factors like fibroblast growth factor (FGF-2), hepatocyte growth factor and transforming growth factor (TGF-β1) [6]. ACS contains high concentration of IL-1Ra, an antagonist to IL-1 that is a biochemical 'sensitizer' of nerve roots in radiculopathy [7]. Hence, ACS is a promising new treatment option for patients with unilateral lumbar radicular compression. The studies on efficacy of epidural perineural ACS in unilateral lumbar radiculopathy are limited. There are no such studies on the Indian population [47]. With this background, we designed a study to evaluate the efficacy of ACS in reducing pain and disability in unilateral lumbar radiculopathy.
Materials and Methods
The study was conducted on patients receiving ACS as a line of treatment at the Orthopedics outpatient department of Post Graduate Institute of Medical Education and Research (Chandigarh) from January 2011 to June 2012. We obtained ethical clearance from the institutional board before the study was initiated. Out of 1,224 patients, 110 male or female patients aged between 30 years to 60 years having moderate to severe low backache with radiation to unilateral lower limb of at least 6 weeks duration as determined by visual analogue scale (VAS) and positive straight leg raising test (SLRT) underwent clinical and radiological investigations. Of the 1,224 patients, 1,114 patients were excluded from the study based on history and clinical examination findings. The exclusion criteria included history of surgery on the lumbar spine and spinal canal stenosis, anticoagulant use and bleeding diathesis or altered coagulation, associated cervical myelopathy, systemic bone or joint illnesses, need of early surgery, epidural steroid injection to the affected nerve root in the prior 3 months, cortisone or opioid use in the prior 6 months, acute trauma, cauda equina syndrome, progressive neurological deficit, motor deficit, pain originating from an infection, allergies to corticosteroids or local anesthetics; and pregnancy and absence of substantial radicular pain as the presenting symptom.
Out of 110, 20 patients with radiological findings of abnormal disc degeneration as determined by X-ray based on Lane et al. [8] grading and magnetic resonance imaging (MRI) based on Pfirrmann et al. [9] grading were included in the study. Ninety patients were excluded based on the radiological findings like multiple disc level, bilateral involvement, canal stenosis, less grade in MRI and X-ray, lack of a radiographically detectable abnormality. We included 20 patients with unilateral lumbar radiculopathy due to disc degeneration (lumbar spondylosis) after informed consent. The mean patient age was 37.15 years and the mean body mass index of the patient was 24.92 kg/m2. We prepared ACS as described by Meijer et al. (Fig. 1) [10].
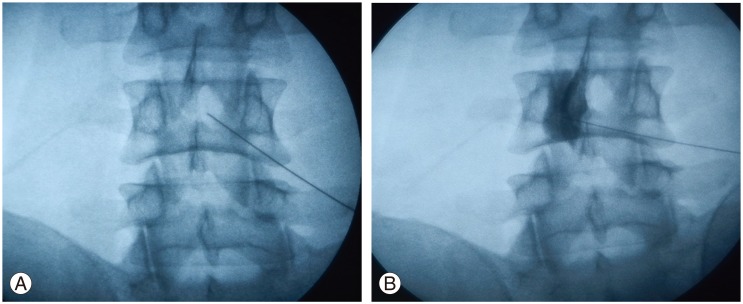
Under biplanar luoroscopic imaging in anterior-posterior and lateral views, ACS was administered through epidural perineural technique (Fig. 2). The entry site was marked with indelible ink. Sterile preparation was performed with alcohol followed by Betadine (povidone-iodine) solution. Local superficial anesthesia was by injection with 1% lidocaine buffered with a small amount of bicarbonate. The introducer needle for passing skin and interspinous ligaments was inserted 1 cm below and 2 cm contralateral, with an angle of 30°-45° to the midline. The 29-gauge needle then passed the ligamentum flavum and reached the lateral part of the anterior epidural space, which was recognized by bony contact. After locating the epidural space, under fluoroscopic guidance, contrast media Iohexol-180 (Omnipaque, GE Healthcare, Cork, Ireland), 2 mL was injected in the epidural space. The fluoroscopic images were obtained to confirm the spread. This step was immediately followed by 2 mL injection of ACS. Real-time fluoroscopy was used to verify that no medication would attain intravascular, subarachnoid, subdural, or intradiscal spread [11].
All the patients were kept under observation and the vitals were noted every 5 minutes for 30 minutes. The patient was advised to report in case of any adverse events. Patients were advised not to use nonsteroidal anti-inflammatory drugs. Oral paracetamol (500 mg, total dissolved solids) was used in case of discomfort. The patient was instructed to maintain a diary and to note when paracetamol was taken. The patient was advised to stop paracetamol (if consuming) 48 hours prior to follow-up assessment. A maximum of three injections at 7 days interval were given according to the clinical response. Twenty patients received an average of two injections, ranging from 1 to 3 injections.
Patients in both groups were evaluated by quadruple VAS subjective pain level or intensity of pain assessed on horizontal 10 cm scale was rated by the patients from 0 (no pain) to 10 (most pain possible), revised Oswestry disability index (RODI), and 12-Item Short Form of Health Survey (SF-12) to assess the level of restriction or inability of the patient to perform an activity in the manner or within range considered normal for a human as a consequence of disease. Patients were evaluated by the above mentioned scores after epidural injections at 3 weeks, 3 months, and 6 months.
Data was analyzed using computer statistical software IBM SPSS ver. 20.0 (IBM Co., Armonk, NY, USA). Data was presented as mean±standard deviation (SD) or median (range) where appropriate. Numerical variables were examined for normality. Intra-group comparisons were done using independent t-test and Student's t-test. Scores and skewed data were analyzed using Mann-Whitney's U-test. Incidence of complications was analyzed by chi-square test. A p-value of <0.05 was considered significant.
Results
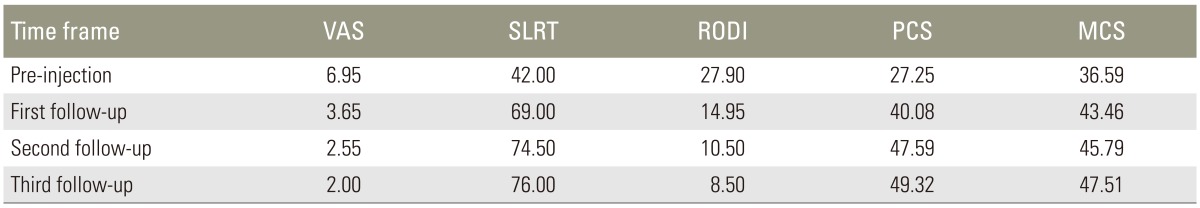
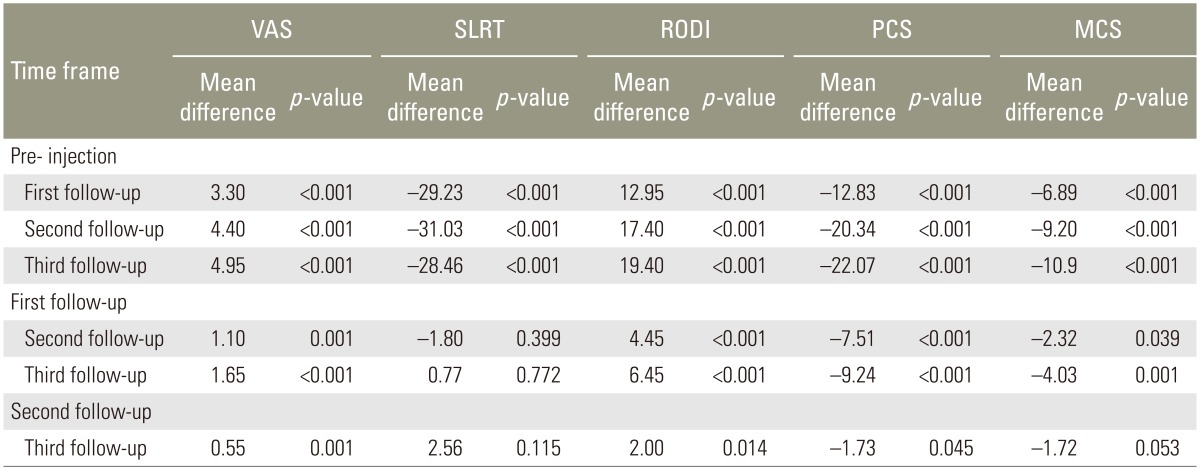
The mean scores of all parameters and statistics at pre-injection and follow-up were given in Table 1. Pairwise comparison of the parameters value at each time frame was shown in Table 2.
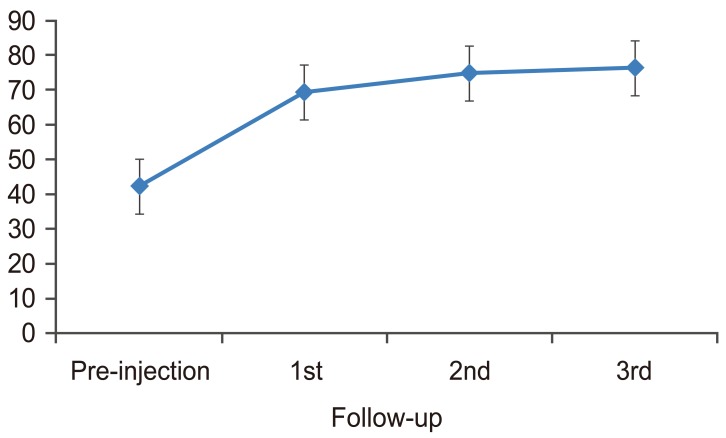
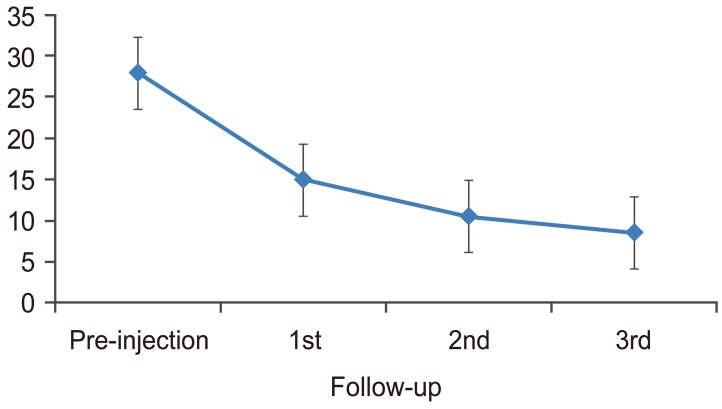
There was a statistically significant change in VAS from pre-injection to first, second, and third follow-up (p<0.001). There was also a significant change from first follow-up to second follow-up (p=0.001) and from second follow-up to third follow-up (p=0.001). The mean VAS decreased from pre-injection to first, second, and third follow-up. The trend was plotted in a graph (Fig. 3). There was a statistically significant change in SLRT scores from pre-injection to first, second, and third follow-up (p<0.001). There was no significant change from first follow-up to second and third follow-up (p=0.399 and 0.772, respectively) and from second follow-up to third follow-up (p=0.115). The mean SLRT scores increased from pre-injection to first, second, and third follow-up. The trend was plotted in a graph (Fig. 4).
In our study, the RODI scores had a statistically significant change from pre-injection to first, second, and third follow-up (p<0.001). There was also significant change from first follow-up to second follow-up (p=0.001) and from second follow-up to third follow-up (p=0.001). The mean RODI scores decreased from pre injection to first, second, and third follow-up. The trend was shown in a graph (Fig. 5).
The SF-12 regarding general health has a total of 12 questions, with each question assigned a value ranging from 0 to 5, with the total score ranging from 0 to 50. Scoring for the physical health component score-12 (PCS-12) and mental health component score-12 (MCS-12) of the SF-12 was performed using the SAS scoring program version 9.1.3 from the Medical Outcomes Trust, Massachusetts, USA. PCS-12 and MCS-12 range from 0 to 100, with greater scores representing better health. Both the PCS-12 and MCS-12 are transformed into T-scores, normalized for the general United States population. The score for an individual or mean score for a group of patients is thus reported relative to a mean of 50.0 and a SD of 10.0 in the general United States population. Consequently, the SF-12 scores for the Indian-based population presented in this study are relative to the general United States population. The scores in the three follow-ups were compared with the pre-injection scores and the trend was studied in two groups to understand the effect of treatment on functional outcome [12].
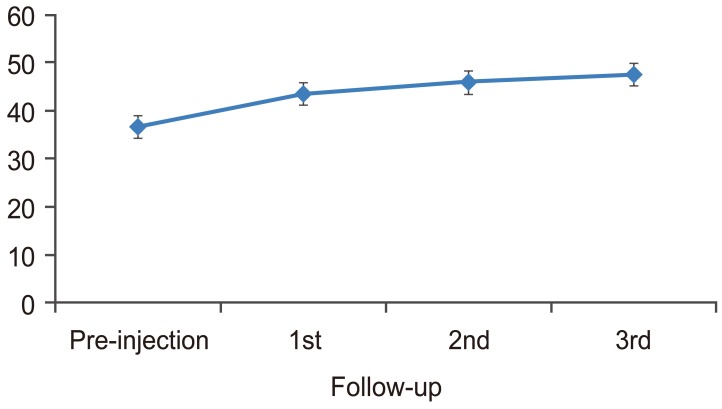
PCS had a significant change from pre-injection to first, second, and third follow-up (p<0.001). There was also significant change from first follow-up to second follow-up (p=0.001) and from second follow-up to third follow-up (p=0.001). The mean PCS increased from pre-injection to first, second, and third follow-ups. The trend was plotted in a graph (Fig. 6). MCS had a significant change from pre-injection to first, second, and third follow-ups (p<0.001). There was no significant change from first follow-up to second follow-up (p=0.039); and no significant change from second follow-up to third follow-up (p=0.053). The mean MCS increased from pre-injection to first, second, and third follow-ups. The trend was plotted in graph (Fig. 7).
Five patients (20%) had complications. Most of the complications were immediate and systemic rather than local and were of short duration lasting less than 30 minutes. The various complications noted were syncope, dizziness, headache, sweating, and tachycardia. None of the complications in either group were of severity or concern and subsided within half an hour when the patients were under observation. One patient had back stiffness after injection for 2 days that subsided spontaneously.
No serious complications such as infection, marked muscle atrophy, deep vein thrombosis, fever, hematoma, tissue hypertrophy, adhesion formation, or other major adverse events occurred among our study subjects. Since ACS is an autologous preparation, the risk of introducing foreign material is effectively eliminated, although the entire procedure must be carried out in sterile conditions. The use of autologous blood products in this manner reduces the risk of transmissible infection and allergic reactions.
Discussion
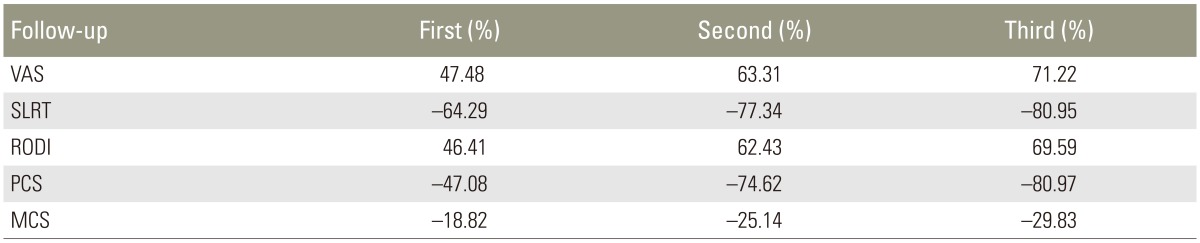
The trends of all parameters were shown in Table 3. The primary outcome measure used in our study was VAS. Mean VAS scores decreased from pre-injection to further follow-ups. The results indicated a decrease in pain by 47.48% at first follow-up, by 63.31% at second follow-up and by 71.22% at third follow-up, as compared to baseline. All group comparisons were statistically significant (p<0.001 in all cases) in the framework of ordered testing (i.e., from time point of first follow-up to time point of third follow-up). The trend noted by Becker et al. [13] was also similar to our findings and showed a slight tendency of worsening in VAS in group with triamcinolone acetate and increase in VAS in the group with ACS scores from second month to sixth month, which was not significant.
There was an improvement in SLRT by 64.29% at first follow-up, by 77.34% at second follow-up and by 80.95% at third follow-up, as compared to baseline. All comparisons were statistically significant (p<0.001 in all cases) in the framework of ordered testing (i.e., from time point of first follow-up to time point of third follow-up). Cocelli et al. [14] compared effectiveness of epidural triamcinolone and betamethasone with SLRT as a primary outcome measure. The pre-injection SLRT was 31.2 degrees and there was a significant improvement in first follow-up that was maintained at further follow-up.
RODI was the secondary outcome measure used in present study. Mean RODI scores showed a decreasing trend in all follow-up evaluation. There was an increased RODI percentage benefit by 46.41% at the first follow-up, by 62.43% at the second follow-up and by 69.59% at the third follow-up, as compared to baseline. Baseline functional capacity in a study by Rados et al. [15] was comparable for both the interlaminar and the transforaminal group (52% vs. 53%, ODI converted into percent) when assessed using the ODI (p=0.647). At 6 months, both groups improved in function with an average of 39% for the interlaminar group and 38% for the transforaminal group, suggesting a change from severe to moderate disability scoring range. Cocelli et al. [14] compared the effectiveness of epidural triamcinolone and betamethasone. They reported that the ODI values were significantly decreased from the second week in both groups. Hence, in the present study, significant improvement of RODI might be attributed to the presence of increased amount of growth factors and use of fluoroscopic guided injection technique.
PCS-12 increased by 47.08% at first follow-up, by 74.62% at second follow-up and by 80.97% at third follow-up, as compared to baseline. MCS-12 increased by 18.82% at first follow-up; by 25.14% at second follow-up, and by 29.83% at third follow-up, as compared to baseline. Birbara et al. [16] studied the efficacy of etoricoxib, a new cyclooxygenase-2 selective inhibitor in the treatment of chronic LBP, and used SF-12 as an outcome measure. Patients who were given etoricoxib did not experience a change from baseline that was significantly greater than placebo.
These studies collectively indicate that anti-inflammatory factors alone are not enough in improving the disability and general health in patients with LBP. Addition of growth factors enhances the biological environment and helps in attaining tissue homeostasis in patients with LBP. Thus, a cocktail of anti-inflammatory factors together with growth factors play a vital role in improving the disability and general health. Hence, ACS can modify disease course in addition to reducing pain, disability and improving general health. Further investigation with larger sample size and longer follow-up is required to support the proposed hypothesis that might revolutionize the management of lumbar radiculopathy.
Limitations of this study were the lack of control group for comparing efficacy and short duration follow-up. This limitation can be overcome by proper design of future studies and the inclusion of post procedure MRI after 6 months to determine whether ACS has an effect on the disease course.
Conclusions
ACS can modify disease course in addition to reducing pain, disability and improving general health. Further studies are needed to confirm the results obtained and to understand the mechanism of the action. Further evaluations also required for long-term symptom improvement or the disease modifying properties of ACS in LBP.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.