Neurological Recovery Pattern in Cervical Spondylotic Myelopathy after Anterior Surgery: A Prospective Study with Literature Review
Article information
Abstract
Study Design
Prospective clinical study.
Purpose
The present study aimed to examine the neurological recovery pattern in cervical spondylotic myelopathy (CSM) after anterior cervical decompression and compare it with the existing reports in the literature.
Overview of Literature
Neurological recovery and regression of myelopathy symptoms is an important factor that determines the outcomes of surgical decompression. The present findings contribute to the literature on the pattern of neurological recovery and patient prognosis with respect to the resolution of myelopathy symptoms after surgery.
Methods
This prospective study was conducted in Government Medical College in Jammu, North India between November 2012 and October 2014, a total of 30 consecutive patients with CSM were included and treated with anterior decompression and stabilization. They were prospectively followed up for 1 year and were evaluated for their neurological recovery pattern. The postoperative outcome was evaluated using the modified Japanese Orthopaedic Association (mJOA) score. The recovery rate was calculated using Hirabayashi’s method. The JOA score was assessed before the operation and postoperatively at 1 week, 2 weeks, 1 month, 3 months, 4 months, 6 months, and 1 year.
Results
The postoperative mJOA score was 0 in the 1st month, 12.90±3.57 in the 3rd month, 13.50±3.55 in the 4th month, 14.63±3.62 in the 6th month, and 14.9±3.24 at the final follow-up of 1 year. The average recovery rate during the 1st month follow-up was 0%, and that during the 3rd month follow-up was 12.91% with a range of 0%–50%. The average recovery rate during the 4th month was 32.5%, with a range of 0%–60%, while that during the 6th month was 72.83%, with a range of 0%–100%. The average recovery rate during the final follow-up of 1 year was 54.3%.
Conclusions
Neurological recovery after surgical decompression starts from the 3rd postoperative month and progresses until the 6th postoperative month; thereafter, it gradually plateaus over the subsequent 6 months until it steadies. Symptom duration is an important factor that requires consideration while determining postoperative neurological recovery.
Introduction
Cervical myelopathy includes a range of symptoms and examination findings, including motor and sensory abnormalities related to cervical spinal cord dysfunction. During the 1950s, classic anatomical studies established cervical spondylosis as a possible etiology for the compression of spinal cord that led to the development of myelopathic symptoms [1]. Currently, the pathophysiology of cervical spondylotic myelopathy (CSM) is considered multifactorial, with a combination of static factors and dynamic factors that cause compression and repetitive injury to the spinal cord and play a role in its etiopathogenesis [2-5]. The symptoms of this disorder are related to the degree of compression of the various spinal cord tracts, identified on the autopsy examinations of CSM patients showing gray matter atrophy, neuronal loss, and white matter demyelination [6,7]. Surgical decompression of the cord is recommended in patients with moderate to severe disease to enhance recovery [8]. Studies have evaluated the outcomes of different treatment methods, such as anterior decompression, laminectomy, and laminoplasty [9-14] and the factors affecting surgical outcomes [15-17]. However, these assessments tend to be of a more global perspective, looking at a composite score for the upper limb, lower limb, as well as bladder and bowel function. Limited evidence is available regarding the pattern of neurological recovery, especially in anterior cervical spine surgery, each presenting some variations in their observation regarding the recovery pattern of the neurological status [18-21]. Studies conducted in India on the neurological recovery pattern are limited to the recovery after the posterior procedure for CSM [22]. The present study observed the neurological recovery pattern in CSM patients after anterior surgical decompression with a review of the existing literature. This information is very helpful for arriving at a reasonable prediction of the recovery course and prognosis in CSM patients after anterior decompression surgery vis-a-vis after posterior decompression surgery.
Materials and Methods
This prospective study was conducted at Government Medical College in Jammu, North India between November 2012 and October 2014 after obtaining the appropriate approvals from the institutional ethical committee (Ref. no., IEC/2015/63). CSM diagnosis was established on the basis of clinical signs and symptoms of cervical myelopathy with radiological correlation on magnetic resonance imaging. Total 30 consecutive CSM patients were enrolled in the study after obtaining the due consent and were treated with anterior cervical decompression and fusion (ACDF) using tricortical iliac crest bone graft with fixation using an anterior cervical locking plate. The anterior Smith Robinson approach was used for ACDF under general anesthesia. The subjects were subsequently followed up for 1 year with regular follow-ups at 1 week, 1 month, 3 months, 4 months, 6 months, and finally at 1 year. The neurological recovery and outcome were recorded using the established scoring system of modified Japanese Orthopaedic Association (mJOA), Nurick grading, and neurological recovery rate using the mJOA.
As per the inclusion criteria, adult patients of either sex, aged 40–75 years, with CSM due to one or two motion segment involvement were enrolled. Patients with associated neurological disorders, such as Parkinson’s disease and hemiplegia; myelopathy due to other degenerative conditions of the spine, such as ossified posterior longitudinal ligament, tumors (intradural and extradural), and infective pathologies like tuberculosis were excluded. Postoperative outcomes were evaluated using the mJOA score (Table 1). The upper limb function score was defined as the upper limb motor JOA score plus the upper limb sensory JOA score, giving a total of 6 points. The lower limb function score was defined as the lower limb motor JOA score plus the lower limb sensory JOA score, giving a total of 6 points. The sphincter function was assessed as per the sphincter function JOA score that has a total of 3 points. The recovery rate was calculated using Hirabayashi’s method.
Recovery rate=(postoperative mJOA score–preoperative mJOA score) / (17-preoperative mJOA score)×100
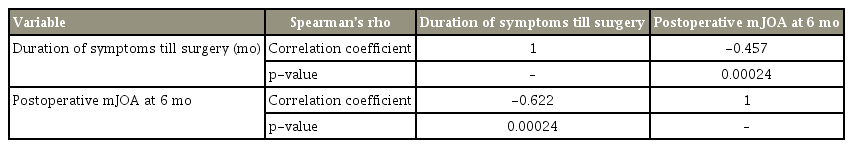
The overall JOA scores, upper limb function JOA score, lower limb function JOA score, and sphincter function JOA score after surgical decompression were documented and analyzed at the regular follow-up. The pattern of neurological recovery was documented using statistical analyses for performing a comparison of the preoperative and postoperative mJOA scores at various time intervals among the subjects using Kruskal-Wallis one-way analysis of variance on ranks (Table 2) and all pairwise multiple comparison procedures (Turkey test) was used (Table 3). A p-value <0.5 was considered statistically significant. A correlation between the duration of preoperative symptoms and the postoperative mJOA score at 6 months was established statistically using Spearman’s rho correlation coefficient (Table 4).
Results
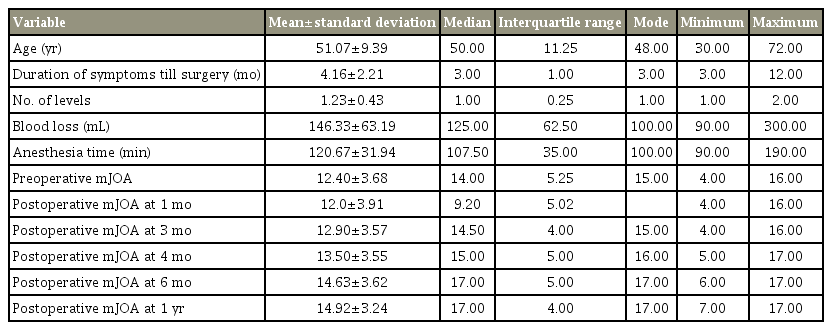
The study included 30 CSM patients, with a mean±standard deviation age of 51.07±9.39 years (range, 30–70 years). Of these 30 patients, 18 (60%) were men and 12 (40%) were women. The most commonly affected level was C5–C6, observed in 12 patients (40%). There were 23 patients (76.67%) with a single level affected, while two levels were affected in the remaining seven patients (23.33%). The mean duration of symptoms at the time of surgery was 4.16±2.21 months (range, 3–12 months). No patient was lost to follow-up at 1 year. The mJOA score was used to access the preoperative disease severity and objective postoperative recovery. The mean preoperative mJOA score was 12.40±3.68 (range, 4–16). Postoperatively, the patients were evaluated at regular follow-ups, and the postoperative mJOA score was recorded at 1 month, 3 months, 4 months, 6 months, and at the final follow-up of 1 year. The postoperative mJOA score was 12.0±3.91 (range, 9–16) in the 1st month, 12.90±3.57 (range, 4–16) in the 3rd month, 13.50±3.55 (range, 5–17) in the 4th month, 14.63±3.62 (range, 6–17) in the 6th month, and 14.9±3.24 (range, 7–17) at the final follow-up at 1 year (Table 5).
The recorded upper limb mJOA score improved from 3.0±1.5 preoperatively to 4.0±1.2 at the final follow-up with an average recovery rate of 45%. Similarly, the lower limb function score improved from 3.1±1.4 preoperatively to 3.9±1.2 at the final follow-up with an average recovery rate of 28%. Seven patients (24%) exhibited postoperative improvement in the sphincter function, indicated by an improvement in the sphincter function JOA score from 2.3±0.7 before the surgery to 2.9±0.8 after the surgery and an average recovery rate of 19%. The pattern of upper limb, lower limb, and sphincter function improvement followed the overall neurological recovery patterns.
1. Duration of symptoms until surgery and postoperative modified Japanese Orthopaedic Association score at 6 months
In our study, the mean symptom duration until the surgery was 3.63±9.39 months, and the mean postoperative mJOA score at 6 months was 14.63±3.62 with a range of 6–17. There was a definite correlation between the symptom duration until surgery, and the postoperative mJOA score at 6 months. This correlation was calculated statistically with Spearman’s rho correlation coefficient, wherein the p-value was 0.015. The correlation between the symptom duration (months) and the postoperative mJOA score at 6 months was statistically significant at a p-value of 0.05 level (two-tailed).
2. Postoperative recovery pattern
The neurological recovery pattern observed in this study was assessed using Tukey test, where the preoperative and postoperative mJOA scores at 1 month, 3 months, 4 months, 6 months, and 1 year were compared with each other. We noted statistically significant differences in the comparisons of the following pairs: preoperative versus 3-month mJOA score, preoperative versus 4-month mJOA score, preoperative versus 6-month mJOA score, 3-month mJOA score versus 4-month mJOA score, 3-month versus 6-month mJOA score, and 4-month versus 6-month mJOA score, with p-values of 0.0023, 0.00001, 0.00001, 0.0077, 0.00001, and 0.00001, respectively, all showing statistical significance, with p-values being <0.05. The 6-month mJOA score and the 1-year mJOA score were not significantly different, with a p-value of 0.456. The preoperative and 1-month mJOA scores were not significantly different.
3. Recovery rate
In order to quantify the recovery pattern during the postoperative period in present study, we calculated the percentage of recovery in the patients using the mJOA recovery rate by applying the Hirabayashi method {recovery rate=(postoperative mJOA score–preoperative mJOA score)÷(17-preoperative mJOA score)×100}. We found this to be a very effective method of quantifying the results. The recovery rate was calculated during the 1-month, 3-month, 4-month, and 6-month follow-ups. The average recovery rate during the 1-month follow-up was 0%, while that during the 3-month follow-up was 12.91%, with a range of 0%–50%. The average recovery rate during the 4-month follow-up was 32.5% with a range of 0%–60%. The average recovery rate during the 6-month follow-up was 72.83% with a range of 0%–100%.
4. Preoperative symptom duration and postoperative modified Japanese Orthopaedic Association score at 6 months
There was a definite correlation between the duration of symptoms until surgery and the postoperative mJOA score at 6 months in our study. This correlation was calculated statistically using Spearman’s rho correlation coefficient, wherein the p-value was 0.015 and scatter diagram, showing the linear relationship between them (Fig. 1). The correlation between the duration of symptoms (months) and the postoperative mJOA score at 6 months was statistically significant at p<0.05 level (two-tailed), as shown in Table 4.
Discussion
Cervical myelopathy is a progressive condition. Following the onset of myelopathic signs and symptoms, there is loss of fine motor activity, instability of gait, and in extreme cases, bowel and bladder incontinence with reduced ambulation, leading to extreme disability [3,4]. Surgical decompression augmented with stabilization is an effective method of halting disease progression and providing chances for the recovery of the compromised spinal cord. A Cochrane review of randomized controlled trials on the role of surgery in mild CSM has concluded that the early results of surgery were superior to those of non-operative treatment in terms of pain, weakness, and sensory loss [13,23]. Surgical decompression using anterior or posterior approaches produces excellent or good results in about 70% of the patients [13,24-30]. Patients with larger transverse area of the cord, younger patients, those with a shorter symptom duration, and those with single rather than multiple levels of involvement are expected to have better prognosis with surgical intervention [2,18,31]. In our study, the mean preoperative symptom duration was 3.63±2.57 months, with a range of 1–12 months. The preoperative symptom duration was correlated to the preoperative mJOA score that determined disease severity. The longer the preoperative symptom duration, the higher the preoperative mJOA score; this ultimately decides the postoperative mJOA score and surgical outcome. Yamazaki et al. [31] have shown that a shorter symptom duration is an important factor that contributes to excellent recovery in elderly patients. Similarly, in the present study, we found a statistically significant correlation between the preoperative symptom duration and the preoperative mJOA scores. We also observed that the less severe the preoperative neurological deficit, better is the postoperative neurological recovery and higher is the postoperative mJOA score.
Irrespective of the selected surgical approach, the goal of operative treatment is the decompression of the spinal cord (while preserving the alignment and stability) with consideration of both the static and dynamic factors. Surgical intervention in patients with moderate to severe myelopathy can be expected to halt symptom progression. The choice between the anterior and posterior approach is debatable with proponents of either approach merit one over the other. Usually, in multilevel cervical compression with preserved lordosis, posterior surgery is preferred over anterior surgery. Anterior surgery is generally performed when the compression is localized to the anterior aspect of the cord and is limited to one or two levels. Although the primary intention of surgical decompression is to halt disease progression; however, recovery has also been observed in several cases. However, few studies have reported on the pattern of neurological recovery, while to our knowledge, none have reported on anterior surgery in an Indian population. The JOA score is commonly used to assess the symptom severity in CSM patients [18,19,31,32] and is used to examine the upper limb, lower limb, and sphincter functions of the patients. The mean recovery rate reported at the final follow-up in various studies varies from 55%–61%. This study observed very little neurological improvement during the initial postoperative period until postoperative 3 months, with significant recovery being noted during 3–6 months that plateaued over the course of 1 year. This indicates no significant improvement in the mJOA score during the first postoperative month. Thus, significant postoperative neurological recovery was noted from postoperative month 3 that gradually improved during 3–6 months after the surgery. These findings were consistent with those published by Cheung et al. [18]. However, Moussellard et al. [19] noted significant recovery as early as after 1 month postoperatively. The JOA score plateau was achieved at 1 year in the current study as compared to that at 6 months, as reported by Cheung et al. [18] and Acharya et al. [22] and at 24 months, as reported by Moussellard et al. [19]. To further add to the variability in the recovery rate plateau, Ren et al. [20] reported a recovery plateau of 9 months.
Further, in contrast to the current findings, Acharya et al. [22] reported biphasic recovery pattern in severe CSM patients treated with laminoplasty. Majority of the recovery reportedly occurs during the first 6 months; however, it is distributed between majorly the first 2 weeks and between 2 weeks and 6 months. This reported early recovery pattern may be attributable to the use of the posterior approach during surgery, and the authors have not performed a statistical comparison of the neurological recovery at various postoperative follow-ups. This underlines the wide variability in the onset of significant recovery patterns and the plateau phase. This should be communicated to the patient during the preoperative discussion, thereby moderating the expectations from the procedure. When viewed individually, the recovery of upper limb function was greater than that of the lower limb and sphincter functions. Neurological recovery was apparently the highest in the upper limb function, followed by that in the lower limb function, and was poorest in the sphincter function [18]; this was similar to the observation by Chiles et al. [24] and Cheung et al. [18]. They reported 75.4% and 65% improvement, respectively, in the upper limb function and 46.7% and 44% improvement, respectively, in the lower limb function. We observed that patients with a higher preoperative mJOA score (>12) had a higher recovery rate during follow-up, indicating that patients with mild disease tend to recover better and have higher chances of neurological recovery during the postoperative period. These findings can help the preoperative prediction of the prognosis in cervical myelopathy patients and help understand the expected neurological recovery after surgical decompression.
Conclusions
The present study is one of the few trials that have examined the pattern of neurological recovery in Indian patients who have undergone anterior surgical decompression for cervical myelopathy; therefore, these findings are an important contribution to the present literature that could enhance the prediction of prognosis and improve neurological recovery after surgery. Our findings show that neurological recovery starts at 3 months after anterior surgical decompression and continues to progress until postoperative 6 months, after which, it gradually plateaus over the subsequent 6 months until it stabilizes. Upper limb neurological function recovery is superior to lower limb function recovery, which in turn is better than bladder function recovery. The preoperative symptom duration is an important determinant for ascertaining the postoperative neurological recovery.
Notes
No potential conflict of interest relevant to this article was reported.