Analysis of the Functional and Radiological Outcomes of Lumbar Decompression without Fusion in Patients with Degenerative Lumbar Scoliosis
Article information
Abstract
Study Design
Retrospective study.
Purpose
This study aimed to analyze the functional and radiological outcomes of lumbar decompression in patients with degenerative lumbar scoliosis (DLS).
Overview of Literature
Patients with DLS have symptoms related to lumbar canal stenosis (LCS) and those due to compensated spinal imbalance. Whether the deformity is the cause of pain or is an adaptive change for the ongoing LCS remains debatable. The extensive surgery for deformity correction along with spinal fusion is reported to have high perioperative morbidity and complication rate.
Methods
This retrospective analysis involved 51 patients who underwent lumbar decompression for LCS associated with DLS from October 2006 to October 2016. The magnitude of the curve was determined using Cobb’s angle and lumbar lordosis (D12–S1) on the preoperative and final follow-up, respectively. The Visual Analog Scale (VAS) and modified Oswestry Disability Index (mODI) scores at the preoperative and final follow-up indicated the functional outcome. Statistical analyses were performed using Student t-test.
Results
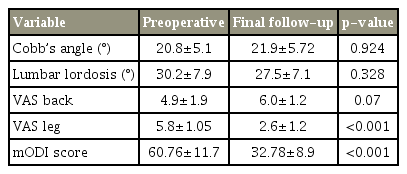
All 51 patients were included in the statistical analyses. The mean patient age at presentation was 63.88±7.21 years. The average follow-up duration was 48±18.10 months. The average change in the Cobb’s angle at the final follow-up was statistically insignificant (1°±1.5°, p=0.924; 20.8°±5.1° vs. 21.9°±5.72°). The mean change in lumbar lordosis at the final follow-up was statistically insignificant (3.29°±1.56°, p=0.328; 30.2°±7.9° vs. 27.5°±7.1°). There was statistically insignificant worsening in the back VAS scores at the final follow-up (4.9±1.9 vs. 6.0±1.2, p=0.07). There was statistically significant improvement in the leg pain component of the VAS score at the final follow-up (5.8±1.05 vs. 2.6±1.2, p<0.001). There was statistically significant improvement in the mODI scores at the final follow-up (p<0.001).
Conclusions
Lumbar decompression in DLS is associated with good functional outcome, especially when the symptoms are related to LCS. Curve progression following lumbar decompression is very less at mid-term and is similar to that in the natural course of the disease.
Introduction
Degenerative lumbar scoliosis is one of the most common disorders in the elderly population with spinal ailments [1]. The prevalence of degenerative lumbar scoliosis is on the rise owing to increasing elderly population [2]. Symptomatic degenerative lumbar scoliosis significantly affects the quality of life in an aging population [1,2]. It is defined as a spinal deformity with a Cobb angle >10° in the coronal plane, occurring in a skeletally mature individual and accompanied by degenerative changes in the intervertebral discs and facet joints of the spine [3]. In addition to spinal deformity, low back pain and radiculopathy resulting from instability and associated lumbar canal stenosis can significantly deteriorate the health related quality of life of an aging population [4,5]. Moreover, contradictory reports exist on whether the deformity is the cause of pain or is an adaptive change for the ongoing lumbar canal stenosis. Compensated imbalance, i.e., different from primary imbalance and presents as an inability to stand upright, develops as an accommodative change for severe lumbar stenosis [6]. Although patients with mild symptoms can be managed conservatively, those with severe radicular pain, disabling back pain, neurological deficits, coronal and sagittal imbalance, and those who do not respond to conservative management require surgery. Decompression without fusion efficiently alleviates the radicular symptoms and neurogenic claudication [7]. Many previous studies have recommended fusion in degenerative lumbar scoliosis owing to the risk of curve progression after decompression alone, causing increased instability, continued back pain, and more neural symptoms [4,8]. However, the extensive surgery for deformity correction along with spinal fusion is reported to have high perioperative morbidity and complication rate resulting in a significant burden on the existing healthcare system [5,9]. In such a scenario, is it rational to apply the dictum “achieving more by doing less?” This study aimed to analyze the functional and radiological outcomes of lumbar decompression in patients with degenerative lumbar scoliosis.
Materials and Methods
This was a retrospective analysis. The clinical information of consecutive patients undergoing lumbar decompression without fusion for lumbar canal stenosis associated with degenerative lumbar scoliosis from June 2006 to May 2016 was analyzed retrospectively. Patients with degenerative lumbar scoliosis >10° along with lumbar canal stenosis, radiculopathy, and significant claudication were enrolled. Patients with a previous history of trauma, osteoporotic vertebral compression fractures, infectious spondylodiscitis, inflammatory disorders, and revision spine surgeries were excluded. All patients involved in this study provided the informed consent. Fifty-one patients who fulfilled the inclusion criteria were included in statistical analyses.
1. Surgical methods
All the patients underwent conservative treatment for an appropriate duration before the surgery. All the patients were operated in a single unit at a single center by a single surgeon and were followed for at least 2 years postoperatively. Before the surgery, all the patients underwent plain as well as dynamic radiography in the standing position. Magnetic resonance imaging (MRI) of the lumbosacral spine along with screening of the whole spine was also performed preoperatively to identify the levels of stenosis. Patients underwent conventional open lumbar laminectomy along with medial facetectomy. The extent of decompression was determined based on preoperative MRI. Whenever possible, an attempt was made to preserve the integrity of the facet joint without compromising on neural decompression by performing undercutting of the medial facets. Operative data in terms of surgical time, blood loss, and any intraoperative complications were recorded. Postoperatively, all the patients were mobilized as per pain tolerance. The patients were discharged 8–10 days after the surgery after adequate mobilization. All the patients were advised the use of orthopedic brace for 2–3 months postoperatively.
2. Radiographic and clinical outcome measurement
All the patients underwent plain standing radiography at each follow-up. The magnitude of the curve was determined using Cobb’s angle in the coronal plane [3], while the sagittal profile was assessed using lumbar lordosis (D12–S1) at the preoperative, 1-year, and final postoperative follow-up.
All the patients were asked to complete the outcome questionnaire at each clinical visit. Pain and disability was scored using the Visual Analog Scale (VAS) for low back pain and leg pain along with the modified Oswestry Disability Index (mODI) at the preoperative, 1-year, and final postoperative follow-up. Depending on the mODI scores, the patients were classified into the minimal disability group (score 0–20), moderate disability group (score 21–40), significant disability group (score 41–60), and crippling back pain group (score >60).
3. Statistical analyses
Statistical analyses were performed for deformity progression in the coronal plane and change in the lumbar lordosis at the preoperative and final follow-up using unpaired Student t-test. Statistical analyses were also performed for the VAS scores for back pain and leg pain and the mODI scores at the preoperative and final follow-up using unpaired Student t-test. A p-value <0.05 was considered to be statistically significant.
Results
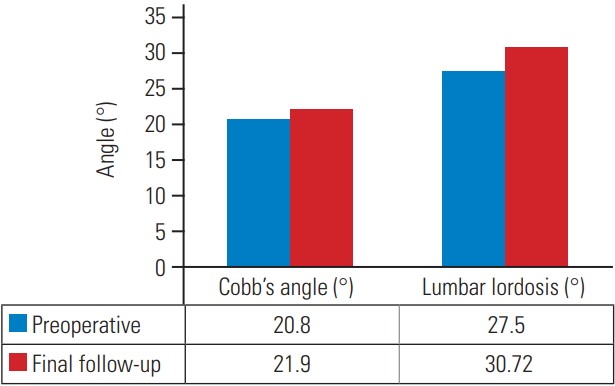
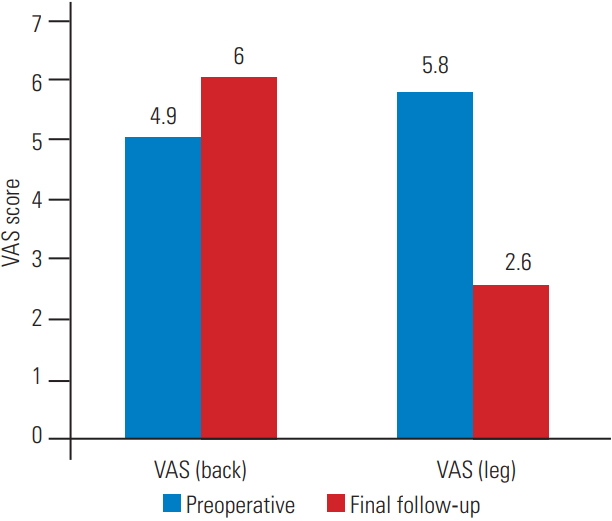
All 51 patients were included in the statistical analysis. The study population included 20 men and 31 women. The mean blood loss was 331±37 mL, and the mean surgical time was 127.6±18.3 minutes. The mean age of patients at presentation was 63.88±7.21 years. The average follow-up duration was 48±18.10 months. The magnitude of deformity, as measured using Cobb’s angle at presentation ranged from 39°–11°. The mean scoliosis angle, as measured using Cobb’s angles at the preoperative, 1-year, and final follow-up was 20.8°±5.1°, 20.8°±5.45°, and 21.9°±5.72°, respectively. The mean change in the Cobb’s angle at the final follow-up was 1°±1.5° compared to that in the preoperative period. This change was statistically insignificant (p=0.924). The mean lumbar lordosis at the preoperative, 1-year, and final follow-up was 30.2°±7.9°, 29°±7.8°, and 27.5°±7.1°, respectively. The average change in the lumbar lordosis at the final follow-up was 3.29°±1.56° compared to that in the preoperative period. This change was statistically insignificant (p=0.328). The mean VAS scores for back pain at the preoperative and final follow-up were 4.9±1.9 and 6.0±1.2. This difference in the scores was statistically insignificant (p=0.07). The average VAS scores for leg pain at the preoperative and final follow-up were 5.8±1.05 and 2.6±1.2, respectively. The difference in the scores was statistically significant (p≤0.001). The average mODI score at the preoperative and final follow-up was 60.76±11.7 and 32.78±8.9. The average change in the mODI score at the final follow-up was 27.88±12.4. The difference in the mean mODI scores was statistically significant (p<0.001). As per the mODI classification, preoperatively, 4/51 patients (7.8%) were mildly disabled, 24/51 (45.1%) were moderately disabled, and 23/51 (45%) were severely disabled. At the final follow-up, 32/51 patients (62.75%) had mild disability, 15/51 (29.41%) had moderate disability, and 4/51 (7.8%) had severe disability. Table 1 summarizes the results, and Figs. 1–3 summarize the graphical representations of the results. All four patients with poor mODI scores developed clinically significant adjacent segment disease. Of these four patients, two were managed non-operatively because radicular pain subsided with selective nerve root blocks. However, the other two patients required reoperation because of recalcitrant radiculopathy and neurological claudication. It is noteworthy that contrary to popular belief, adjacent segment disease can also occur following lumbar decompression only. However, further larger randomized controlled studies are required to confirm this.
Discussion
Degenerative lumbar scoliosis is defined as the coronal curvature developing in a normal lumbar spine due to degeneration of intervertebral discs and facet joints [10]. The prevalence of degenerative scoliosis in the general population is 6%–68% [1,11,12]. However, with the aging population leading an active life and increase in the life expectancy, there is a rise in the number of patients with degenerative lumbar scoliosis [5]. Most patients with degenerative lumbar scoliosis are mildly symptomatic and are managed with non-operative treatment.
Symptomatic degenerative scoliosis has a significant impact on the quality of life of the aging population [13]. In addition to spinal imbalance, patients with degenerative lumbar scoliosis also exhibit symptoms arising due to degenerative lumbar canal stenosis, mainly neurological claudication, radiculopathy, or weakness due to neural compression [14]. In a prospective analysis on adult patients diagnosed with adult scoliosis, Schwab et al. [15] clearly demonstrated that adult scoliosis (either adolescent-onset idiopathic or de-novo degenerative scoliosis) has a significantly greater impact on the 36-item Short-Form Health Survey (SF-36) scores as compared to other morbid conditions, such as hypertension and back pain. In a prospective analysis of a multicenter international database collected from eight industrialized countries across three continents, Pellise et al. [16] compared the SF-36 score of patients with adult degenerative scoliosis and those with four other comorbid conditions. Authors have reported that the global burden of degenerative scoliosis is considerably larger than that of other self-reported chronic conditions in the general population.
Many patients are diagnosed with degenerative scoliosis incidentally, either during routine clinical examination or when the deformity is noticed on radiographs performed for diagnosing other diseases. The symptoms arising from degenerative scoliosis could either be associated to spinal deformity or lumbar canal stenosis [14]. Asymptomatic patients are managed conservatively; however, these patients are monitored regularly using periodic radiographs for any deformity progression [7]. Non-operative treatment often involves the use of nonsteroidal anti-inflammatory drugs, physical exercises, aquatic therapy, and yoga [5,9,17]. Lumbosacral orthoses help in relieving pain in the short-term; however, in the long-term, they result in muscle deconditioning and fail to halt curve progression [5]. Facet injections and selective nerve root blocks have both, diagnostic and therapeutic value, especially for locating the pain source [5,9]. Patients experiencing progression of symptoms despite non-operative treatments will require surgical intervention [5].
The results of surgical treatment for lumbar degenerative scoliosis are variable because the etiology is heterogeneous and multifactorial [18]. Apart from social and psychological factors, the choice of surgical treatment depends on the patient age, life expectancy, and medical comorbidities [19]. There are many pertinent questions that arise while deciding the surgical plan for patients with degenerative lumbar scoliosis. Do all patients need fusion? What type of fusion is required, a long fusion or a short fusion? Is there a need at all for deformity correction or does the patient require fusion in situ?
Depending on the nature of the patient’s pain, symptoms, and medical conditions, Silva and Lenke [6] have suggested various surgical options for treating degenerative lumbar scoliosis. As per them, predominant radicular pain is correlated to lumbar canal stenosis and requires decompression with or without fusion. However, predominant axial back pain is strongly correlated with sagittal imbalance, and thus requires long fusion. The role of decompression alone in patients with degenerative scoliosis has been described adequately in previous studies [7,20,21]. In a retrospective analysis of 85 patients with degenerative scoliosis, Transfeldt et al. [13] reported on 21 patients undergoing lumbar decompression. The ODI score at the final follow-up was 39.5±17.7. The mean change in the ODI scores was statistically significant at −7.9±12.4. In the present study, all 51 patients underwent lumbar decompression and had a good and statistically significant functional outcome. The mODI score at the final follow-up in the present study was 32.78±7.9. The mean change in the mODI scores was −27.88±12.4 and was statistically significant (p<0.001). Functional outcome was also assessed in the present study using the VAS for leg pain and back pain. There was statistically significant improvement in mean VAS scores for leg pain at preoperative and final follow-up period (5.8±1.05 versus 2.6±1.2, p<0.001).
Although there was slight clinical worsening in the mean back pain scores at the final follow-up (4.9±1.9 versus 6.0±1.2), this was statistically insignificant (p=0.07) This worsening in back pain can be attributed to the ongoing facet arthritis or to muscle denervation owing to long surgical wounds.
Previous studies have proved that decompression procedures that comprise laminectomy provide short-term pain relief; however, they have no effect on curve progression, instability, and axial back pain [5,7]. The deformity may increase with time and may require a second operation [4,7]. In the present study, Cobb’s angle and lumbar lordosis were used to document and monitor the change in the deformity. The mean change in Cobb’s angle at the final follow-up was 1°±1.5° compared to that in the preoperative period. This was statistically insignificant (p=0.924). This change in Cobb’s angle was similar to that reported by Matsumura et al. [22] but was lower than that reported by Hosogane et al. [23] and Daubs et al. [24]. It is noteworthy that the progression of deformity in the present study (as monitored by Cobb’s angle) was similar to that reported in the natural history studies of degenerative lumbar scoliosis [25,26]. Although our study is not a longterm trial, this may put the notion that decompression accentuates the deformity progression under the question. A long-term study on this subject would be helpful.
The mean change in the lumbar lordosis at the final follow-up was −3.29°±1.56° compared to that in the preoperative period. In contrast to the report by Trenfeldt et al. [13] where patients showed no change in lumbar lordosis at the final follow-up, patients in the present study had statistically insignificant loss of lumbar lordosis (p=0.328).
Authors would like to describe couple of patients from the present study. First patient (Fig. 4) was a 69-year-old female, having left sided degenerative lumbar scoliosis with L3–S1 lumbar canal stenosis with L4–L5 grade 1 degenerative listhesis. She underwent L3–S1 lumbar decompression. At 4-year and 10-month follow-up, she had significant reduction in VAS score for leg pain (9 versus 1). Her VAS score for back pain has also improved (6 versus 4). There was significant improvement in mODI score as well (72 versus 24). The Cobb’s angle had not changed significantly at last follow-up (22° versus 24°). Lumbar lordosis decreased by only 3° at final follow-up (31° versus 28°). Second patient (Fig. 5) was 70-year-old male having left sided degenerative scoliosis along with L1–S1 lumbar canal stenosis with L4–L5 grade 1 degenerative listhesis. He underwent L1–S1 lumbar decompression. At 4-year and 4-month follow-up, his Cobb’s angle increased by 3° (18° versus 21°) while lumbar lordosis decreased by 2° (21° versus 19°). There was significant improvement in VAS leg score (7 versus 2) and mODI score (59 versus 20). However, VAS back score remained unchanged at final followup (3 versus 3).
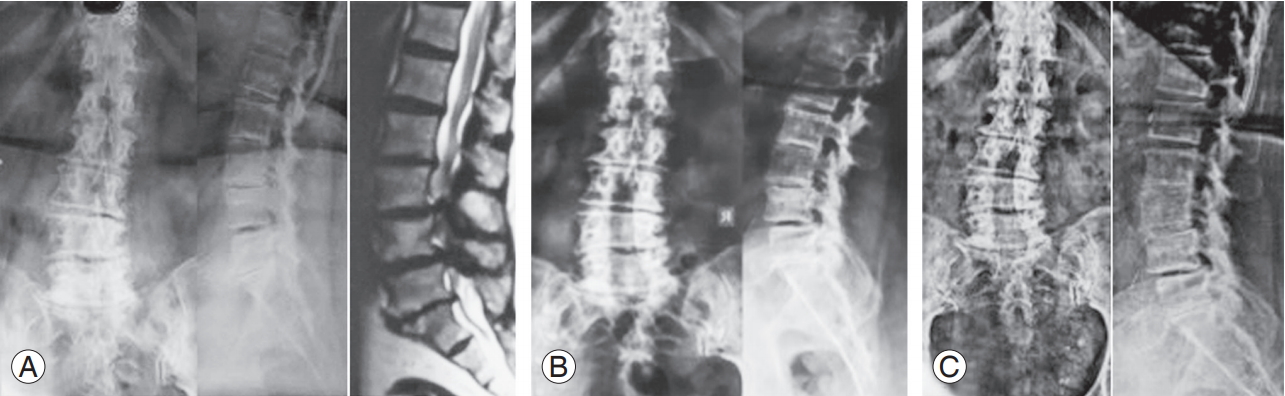
(A) Preoperative AP and lateral radiography and magnetic resonance imaging. Cobb’s angle 18°. (B) AP and lateral radiography at 1 year postoperatively. Cobb’s angle 18°. (C) AP and lateral radiography at 4 years 10 months postoperatively. Cobb’s angle 18°. AP, anteroposterior.
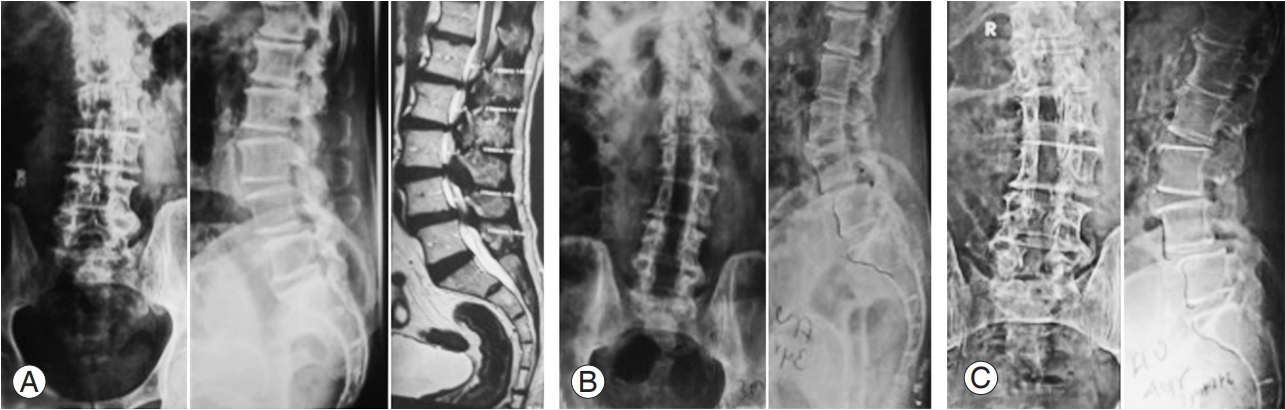
(A) Preoperative AP and lateral radiography and magnetic resonance imaging. Cobb’s angle 12°. (B) AP and lateral radiography at 3 years 1 months postoperatively. Cobb’s angle 18°. (C) AP and lateral radiography at 4 years 4 months postoperatively. Cobb’s angle 18°. AP, anteroposterior.
Recently, many studies have suggested that degenerative lumbar scoliosis patients who undergo decompression surgery with deformity correction and instrumented fusion have good functional outcome in terms of pain relief, patient satisfaction, and walking ability. This is true for severe deformities (curves >30°). As per the advocates of fusion, severe deformities progress with time after decompression alone, especially in the presence of sagittal and coronal imbalance. However, these extensive surgeries of deformity correction in degenerative lumbar scoliosis often involve severe complications, including infections, pseudoarthrosis, cerebrospinal fluid fistulas, myocardial infarction, pulmonary emboli, adult respiratory distress syndrome, implant failure, urinary tract infections, adjacent level compression fractures, post-junctional kyphosis, and high rate of revision surgeries [1,5,9]. The rates of complications are reported to range from 20%–68% [7,27]. Pseudoarthrosis is reported in around 5% of the patients, while adjacent segment disease was reported in almost 30% patients [27]. Implant-related complications in the early postoperative period are reported in about 9% of all patients [28]. Many recent reports have highlighted the increased association of post-junctional kyphosis with deformity correction in patients with degenerative lumbar scoliosis. This incidence may increase further with the inclusion of posterior corrective osteotomies. In an unpublished series by Januszewski et al. [29], 35.5% patients developed post-junctional kyphosis. Moreover, this problem increased with the inclusion of posterior corrective osteotomies (46.2% versus 27.8%).
Degenerative scoliosis is often more rigid than adolescent scoliosis; therefore, it requires a significant amount of release (of ligamentum flavum and facet capsules). It is a well-known fact that these extensive surgeries involve significant blood loss and can sometimes be fatal in the presence of poor nutrition and medical co-morbidities [27]. The presence of anterior disc osteophyte complex sometimes requires additional release via the anterior retroperitoneal approach that may add another set of complications, such as injury to great vessels and visceral organs, retrograde ejaculation, and paralytic ileus [30].
In addition to the problems related to medical comorbidities and surgical approach, another issue that affects these surgeries is that of implant-related complications [27]. Often these patients are osteoporotic (especially post-menopausal women) and implant failure is always a risk, especially with long rigid fixations [5,9,31]. Postjunctional kyphosis is common in these patients above and below the levels of fusion [5]. The risk of pseudoarthrosis in patients undergoing lumbar degenerative scoliosis is around 24% and is maximum and lumbo-sacral junction [32]. In the present study, the rate of complications was 11.76% (6/51). Two patients had minor surgical wound breakdown leading to delayed healing, and two experienced adjacent segment disease causing recurrence of radicular complaints. Other two patients had osteoporotic fractures that required fusion.
Similar to many previous studies, the present study has raised several questions. The foremost query that requires further long-term studies is regarding the deformity progression following lumbar decompression in patients with degenerative scoliosis. Authors believe that as per the Kirkaldy-Willis hypothesis, a degenerative spine eventually lands in a phase of autostabilisation and the progression of deformity would be less functional consequences. The severity of the deformity in the present study population was less than that in the study by Transfeldt et al. [13] (20.8° versus 25°). Thus, the symptoms of axial back pain may not be related to deformity but to facet arthropathy. Moreover, the mean Cobb’s angle of the curve in the present subset of population is very low. The present results cannot be extrapolated to patients with severe deformities at the presentation where the driving force for the symptoms is spinal mal-alignment.
The present study did not aim to establish the supremacy of decompression over fusion in patients with lumbar degenerative scoliosis, but to report on the results of lumbar decompression and study the mid-term radiological and functional outcomes, especially in cases when the symptoms are predominantly related to neural compression and when the spinal deformity is not the main cause for the symptoms.
Moreover, with the current healthcare scenario involving scarcity of resources in many developing countries, it is advisable that healthcare providers impart the appropriate but cost-effective treatment. Surgical complications and revision surgeries have a negative impact on the patient’s quality of life and pose a huge financial burden. The dictum of “achieving more with doing less” may hold true in the scenario described in the present study. A treating physician needs to consider the financial aspect while deciding the surgical treatment for patients with degenerative lumbar scoliosis. To our knowledge, this is the first study from the Indian subcontinent to report on the midterm results of lumbar decompression in adult degenerative scoliosis.
Conclusions
Lumbar decompression in patients with degenerative lumbar scoliosis is associated with good functional outcome, especially when the symptoms are related to lumbar canal stenosis. There can be statistically insignificant increase in back pain following lumbar decompression in these patients. Progression of deformity following lumbar decompression is negligible and is similar to that observed in the natural course of degenerative lumbar scoliosis.
Notes
No potential conflict of interest relevant to this article was reported.