Minimally Invasive Spine Surgery: Techniques, Technologies, and Indications
Article information
Abstract
Over the past few decades, interest in minimally invasive spine surgery (MISS) has increased tremendously due to its core principle of minimizing approach-related injury while providing outcomes similar to traditional open spine procedures. With technical and technological advancements, MISS has expanded its utility not only to simple spinal stenosis, but also to complex spinal pathologies such as metastasis, trauma, or adult spinal deformity. In this article, we review the techniques and technology in MISS and discuss the indications, benefits, and limitations of MISS.
Introduction
The popularity of minimally invasive spine surgery (MISS) has increased along with tremendous advancement in surgical techniques and technologies in recent decades [1]. With its aim of minimizing surgical morbidity and achieving the same surgical outcomes as traditional open spine procedures, MISS is advocated when possible to avoid excessive approach-related injury and subsequent preservation of the normal anatomy while allowing rapid recovery as well as a better quality of life [1-4]. Many studies have reported that patients treated with MISS experienced lower intraoperative and perioperative adverse outcomes, shorter operative time, and a faster return to work [4-6].
Since lumbar microdiscectomy revolutionarily began the employment of MISS in place of open lumbar discectomy, the evolution of modern spine technologies such as endoscopy, navigation, and robotics have expanded the MISS horizon, making it applicable to many complex spine pathologies [1,2,7]. For example, the feasibility and safety of not only simple neural decompression using a microscope or endoscope, but also MISS for treating spine metastasis and spine trauma have been reported [2,7]. Extensive development of biomaterials for spinal implants accounts for the feasibility of MISS in various types of spine pathologies. Improved navigation and robotics technologies allow pedicle screw fixation to be highly accurate, thereby improving the safety of MISS [8]. This paper briefly reviews the techniques and technologies that advance MISS, as well as the various spine pathologies that can be treated with MISS.
Minimally Invasive Spine Surgery Techniques
1. Endoscope-assisted spinal surgery
For the treatment of herniated disc and spinal stenosis, specifically in the lumbar spine, endoscope-assisted neural decompression provides advantages over conventional open surgery. Advantages of endoscope-assisted spinal surgery include less approach-related trauma, preservation of epidural blood supply, and reduced epidural scarring and fibrosis [9]. Along with these advantages, endoscope-assisted spinal surgery can be performed under monitored anesthesia care in an outpatient setting, which potentially reduces the patient’s hospital costs [10]. Endoscope-assisted lumbar surgery is commonly performed for the treatment of herniated discs, spinal stenosis, and infectious spondylitis. Meanwhile, cauda equina syndrome, some large herniated discs, weakness without radiculopathy, and severe fibrotic adhesion due to previous operation are not suitable for endoscopic surgery [10,11]. Favorable clinical and surgical outcomes of endoscope-assisted spinal surgery have been obtained for carefully selected patients in several studies [12-14].
Endoscopic-assisted spinal surgery can be classified based on endoscopic properties: full endoscopic, microendoscopic, and biportal endoscopic [11] (Fig. 1A). Surgeons should choose an appropriate type of endoscopic surgery with careful patient selection. Endoscope-assisted spinal surgery can be performed using a transforaminal approach and an interlaminar approach in the lumbar spine. The most representative examples of endoscopeassisted spinal surgery are interlaminar decompressive laminectomy using uniportal or biportal endoscopy for the treatment of a lumbar stenosis; transforaminal discectomy or foraminotomy using uniportal or biportal endoscopy for the treatment of a lumbar herniated disc; and endoscope-assisted lateral lumbar interbody fusion (LLIF) or transforaminal lumbar interbody fusion (TLIF) for the treatment of degenerative spondylolisthesis, or for central stenosis with spinal instability or with concomitant foraminal stenosis [12-17] (Fig. 1B).

(A, B) Operative field of biportal endoscopic spine surgery. The surgeon is a right-handed person. (C) Intraoperative endoscopic view showing decompressed dura of the ipsilateral side.
One thing to consider when choosing endoscopic surgical procedures is that these procedures require surgical expertise with a steep learning curve [10,18,19]. This steep learning curve arises from the absence of tactile sensation and difficulty obtaining anatomic orientation due to the narrow surgical corridor. Continued surgical experience can reduce the operation-related complications while improving clinical outcomes. Also, for young surgeons, recently introduced biportal endoscopy can be a feasible option, given it offers a wider field of view [20].
2. Minimally invasive transforaminal approach for interbody fusion
The TLIF approach was designed to overcome the limitations of traditional posterior lumbar interbody fusions, which are mainly associated with extensive root and thecal sac retraction and potentially resulting in complications. The TLIF approach involves direct, unilateral access to the disc space by opening the neural foramen; thus, it is possible to efficiently approach the posterior structures. It also can preserve the ligamentous structures as well as the lamina and facet joint [21,22].
Minimally invasive TLIF (MI-TLIF) modifies traditional open TLIF by reducing its working zone, using a tubular retractor and microscope. For MI-TLIF, a 1-cm incision is typically made under fluoroscopy at the lateral aspect of the pedicle behind the facet joint of the surgical level [23]. Percutaneous unilateral or bilateral pedicle screw fixation is commonly performed for MI-TLIF. MITLIF can be applied for a wide range of indications, such as spondylolisthesis, herniated discs, spinal stenosis, or other degenerative lumbar disorders [15,21,22]. It is considered to be more suitable for single-level spine pathologies, especially for foraminal stenosis, central stenosis, or recurrent herniated discs [22].
Several studies have focused on comparing surgical and clinical outcomes of MI-TLIF with those of a traditional open TLIF. Wu et al. [24] reported that MI-TLIF and open TLIF had a similar fusion rate in their meta-analysis. Complications related to surgery were statistically similar but had a tendency to decrease toward MI-TLIF [24]. MITLIF might be associated with faster recovery and shorter operation time, as well as reduced blood loss. Although some studies have reported advantages of MI-TLIF over open surgery, others indicated unfavorable long-term outcomes of MI-TLIF. Jin-Tao et al. [21] had reported that, in the long-term, MI-TLIF did not show considerable advantages over open TLIF, with higher readmission/reoperation rates, and noting MI-TLIF’s steep learning curve. Further long-term studies should be performed to address the effectiveness of MI-TLIF.
MI-TLIF is a technically challenging procedure, especially for an inexperienced surgeon, due to the small working space limited by the tubular retractor. A few studies have demonstrated a steep MI-TLIF learning curve; however, this challenge might be overcome by a substantial length of surgical experience. Additionally, with the aid of endoscopic instruments, surgeons can minimize surgery-related complications, thus improving the safety of MI-TLIF [12].
3. Minimally invasive lateral approach for interbody fusion
Since a retroperitoneal, transpsoas approach to the lateral aspect of the lumbar spine was first described in 2001, LLIF has been modified into similar approaches: oblique lumbar interbody fusion, direct lateral interbody fusion, and extreme lateral interbody fusion (XLIF). These approaches make it possible to laterally access the anterior and mid-column spine from T12–L5 [22,25]. Although the L5–S1 level is not typically accessible due to the iliac crest, the iliac vasculature, and the lumbar plexus, accessibility to multiple levels of the spine makes LLIF advantageous for sagittal and coronal adult spinal deformity, especially with lateral listhesis, and adjacent spinal disorders [26]. Severe central canal stenosis, foraminal stenosis due to lateral bony spur, and severe spondylolisthesis are not suitable indications for LLIF [22] (Fig. 2). Preoperatively, surgeons should carefully review the patient’s anteroposterior and lateral plain radiographs, computed tomography (CT), and magnetic resonance imaging to evaluate spinal pathologies and optimal surgical planning for LLIF. The iliac crest could obscure the disc space, and the abdominopelvic vessel position might be vulnerable to approachrelated injury. Additionally, LLIF might not be suitable for patients who have experienced previous retroperitoneal operation.
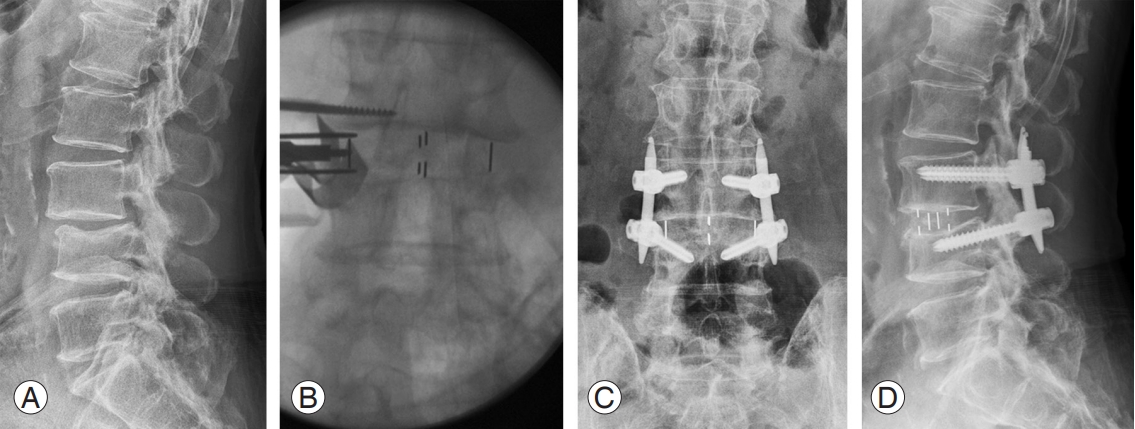
Oblique lumbar interbody fusion in a 62-male patient. (A) Preoperative lateral radiograph showing L3–4 degenerative spondylolisthesis. (B) Intraoperative fluoroscopy-assisted cage insertion. (C, D) Postoperative anteroposterior and lateral radiographs.
Studies have reported an LLIF fusion rate of more than 90% [2,22,27]. Keorochana et al. [28] had reported no significant difference in the fusion rate between LLIF and MI-TLIF, whereas there was a slightly higher complication rate and lower clinical outcomes in terms of Oswestry Disability Index and back/leg pain visual analog scale pain score. Joseph et al. [29] performed a systemic review of 54 studies, reporting that LLIF showed overall higher complication rates with inadequate decompression, graft subsidence, and postoperative neurologic deficit. Employing intraoperative neuromonitoring in LLIF and XLIF could be helpful for reducing intraoperative or postoperative neurologic deficit [30].
Minimally Invasive Spine Surgery Technology
1. Endoscopy
The most important principle of MISS is to save normal vertebral structures. For this reason, many surgeons have attempted to make a smaller surgical corridor by designing surgical instruments such as tubular retractors for MITLIF or by developing a new approach strategy, such as LLIF or XLIF. Among the most important technological developments is employing an endoscope in spine surgery. After several pioneers of endoscope-assisted spinal surgery adopted the innovative application of a laparoscope for minimally invasive procedures, endoscopic spinal surgery became a common part of the diagnostic and therapeutic procedures employed within the field of spine surgery [31].
Development of a high-resolution endoscopic camera system and other endoscopic instruments has permitted a precise and expanded visualization of the surgical field. A current spinal endoscope system offers an expanded field of view angle of 0° to 90°, and thus provides direct visualization of all spine regions, such as the subarticular, far lateral, foraminal, and even intradiscal area if it is used with multiple approaches [32].
It allows the feasibility of endoscope-assisted spinal surgery for treating various types of spine pathologies even through a smaller surgical corridor. The safety and efficiency of endoscope-assisted spinal surgery is expected to increase through the development of innovative technology.
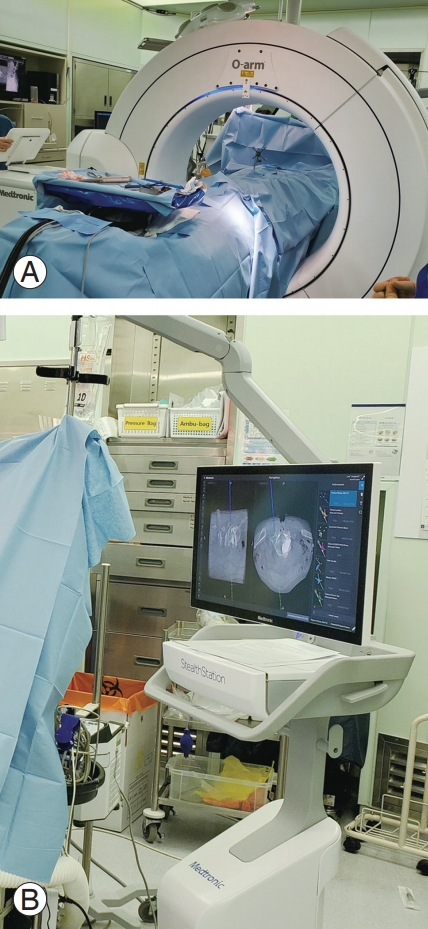
2. Navigation
Within the field of spine surgery, real-time image guidance and navigation systems have provided a potential for improving safety and accuracy of spine surgery over the traditional techniques. High-quality registration of the CT images and utilities of stereotactic three-dimensional (3D) cameras allow intraoperative 3D mapping of the spine as well as real-time anatomic tracking of the instruments (Fig. 3). At first, spine surgeons applied navigation systems to percutaneous pedicle screw fixation. In most cases, percutaneous pedicle screw fixation in MISS has been performed using intraoperative fluoroscopy. Even though fluoroscopy-guided pedicle screw fixation has demonstrated high accuracy, radiation exposure to surgeons and patients has been an issue. The application of CT-based navigation systems might reduce the risk of radiation exposures to surgeons and patients by more than 90% during the procedures while providing much higher accuracy for pedicle screw fixation [3,33,34]. Given MISS, especially MI-TLIF and MI-LLIF, are typically performed with percutaneous pedicle screw fixation, the application of navigation systems provides high reliability and safety. Many studies have reported that the current CT image-based navigation systems are highly reliable and thus can be applied to most MISSs to reduce operation-related complications, including reoperation rates [3,34]. One remaining problem is the navigation system cost, which should be balanced out as its usage rate increases.
3. Robotics
The employment of robotics systems has increased in the field of spine surgery, given they provide precise, reliable, and effective procedures that can be performed quickly [3,34,35]. In combination with a navigation system, robotics systems theoretically promise more accurate pedicle screw fixation and less soft tissue damage. Pedicle screw fixation is a representative application of robotics systems. Although there are insufficient clinical data regarding the utility of robotics systems in spine surgery, several studies have reported that the accuracy of pedicle screw fixation by robotics systems is superior to that of free-hand as well as fluoroscopy-guided pedicle screw fixation [36,37]. One of the most important advantages of robotic spine surgery is that it can overcome the mental and physical fatigue of the surgeon during the procedures [4,34], which could provide better clinical and surgical outcomes. Unfortunately, robotics systems are rarely applied due to their high cost. However, as the costs decrease, the application of robotics systems in spine surgery is expected to increase.
Minimally Invasive Spine Surgery for Complex Spine Pathology
1. Spine metastasis
Due to the advancement of systemic therapy, many patients with spine metastasis live longer. Thus, surgery for spinal metastasis is subject to prior chemotherapy or prior radiotherapy, adding to the patients’ medical fragility in addition to all the risks of non-oncological spine surgery. Optimizing surgery for patients with spine metastasis is essential to prevent postoperative morbidity. With technological advancement, MISS for spine metastasis has become feasible, and thus has been considered as a new treatment option over traditional open surgery [38]. Several studies have reported equivalent functional outcomes and faster recovery with reduced morbidity, such as blood loss and surgical site infection [3,39-42].
In 2019, Barzilai et al. [39] suggested a treatment algorithm for spine metastasis, which consisted of following: patient evaluation using the health-related quality of life questionnaire; cancer-related spinal instability; epidural spinal cord compression; tumor biology; primary or adjuvant radiotherapy; and spinal surgeries such as separation surgery, minimal access surgery, and stabilization. Given the careful patient selection, MISS for spine metastasis significantly reduces surgery-related morbidity and improves patients’ quality of life compared with traditional open surgery.
2. Deformity correction
The use of and indications for MISS are gradually increasing in spine deformity surgery. Compared with traditional thoracolumbar open surgery for deformity correction, minimally invasive surgical approaches provide benefits to reduce approach-related tissue injury, intraoperative blood loss, and surgical site infection [6,43]. Several studies have reported favorable surgical MISS outcomes for treatment of adult spinal deformities as well as adolescent idiopathic scoliosis and neuromuscular scoliosis [3,43,44]. The authors corroborated the feasibility of posterior MISS for deformity correction, MI-TLIF, and MI-LLIF, by which it was possible to obtain sufficient correction of Cobb’s angle and pelvic obliquity [3,7,25,44,45]. Overall, MISS for deformity correction appears to have a comparable surgical outcome to traditional open surgery, while reducing serious complications.
3. Spinal trauma
Given the main goal of MISS is to reduce approachrelated morbidity, MISS for spinal trauma can be a good option in terms of reducing patients’ physiological burden. Fewer approach-related soft tissue injuries, reduced blood loss, and a faster recovery time make it suitable for treating spine trauma patients, who typically also experience other types of trauma. The most important aspects to consider are the appropriate indications. Several factors should be evaluated: the injury pattern, the presence of neurologic deficit, the patient’s medical condition, and the operator’s surgical skill [46]. Several studies have reported their use of MISS for the treatment of unstable thoracolumbar fracture with or without spinal cord injury; flexion- and extension-distraction injuries; and complex sacral fractures [46-49]. Although the necessity of spinal fusion for the treatment of spinal trauma is still controversial, fixation without fusion is considered to be effective in providing stability and sagittal balance for the treatment of thoracolumbar burst fractures without neurologic deficit. In case of incomplete neurologic deficits, lateral MISS accompanied by corpectomy and fusion with cages and pedicle screw fixation are considered an effective treatment option [50].
Conclusions
The main goal of MISS is to minimize approach-related soft tissue injury and to preserve normal anatomy, which permit a better quality of life through faster postoperative recovery. Over the past few decades, significant technological and technical advancements have made this goal possible. Thanks to navigation and robotics systems, safe pedicle screw fixation has become possible. Endoscopic techniques have expanded the visual field of anatomy enough to perform accurate procedures. The development of various surgical approaches, such as transforaminal and lateral approaches, allow various ways of performing minimally invasive decompression and fusion surgeries, from a simple single-level herniated disc to complex spinal pathologies such as adult spinal deformity and spinal metastasis. With the shift toward MISS from open surgery, we expect continuing development of technologies and innovative surgical procedures, giving more patients the option of MISS.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
JP carried out the literature survey and wrote the manuscript; DWH and BTK carried out the literature survey; SMP participated in the design and had primary responsibility for the final manuscript; HJK and JSY helped to revise the manuscript; and all authors read and approved the final manuscript.