Comparative Cohort Study for Expansion of Lateral Recess and Facet Joint Injury after Biportal Endoscopic Ipsilateral Decompression and Contralateral Decompression
Article information
Abstract
Study Design
This was a retrospective longitudinal study of patients operated on consecutively in a single center from May to October 2019.
Purpose
The aim in biportal interlaminar endoscopic decompression surgery for lumbar stenosis is to compare the clinical and radiological outcome of lateral recess decompression and facet preservation, employing ipsilateral (IL) versus contralateral (CL) approaches.
Overview of Literature
There is scant literature comparing the radiological outcome of lateral recess decompression and facet preservation via IL versus CL approaches in patients undergoing biportal interlaminar endoscopic decompression surgery.
Methods
In this retrospective study, we reviewed 37 IL and 34 CL approaches. Postoperative magnetic resonance imaging of the segment involved was carried out on the same day as the operation for comparison with preoperative imaging. Radiological assessments of recess angle, recess height, facet length, and recess dural sac diameters were compared. In addition, pre- and postoperative Visual Analog Scale (VAS) pain scores for the lower limb were analyzed.
Results
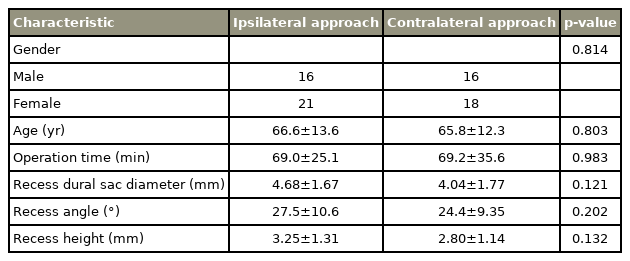
For IL versus CL approaches, we observed statistical differences in the postoperative recess angle (36.0° vs. 43.7°), recess height (4.27 vs. 5.06 mm), and the dural sac expansion ratio for recess diameter (1.54 vs. 2.17). There was better preservation of facet length in the CL approach than in the IL approach (91.9% vs. 83.7%). There was no difference in VAS improvement between the groups (69.3% vs. 63.6%).
Conclusions
Unilateral biportal decompression via the CL interlaminar approach may offer better lateral recess clearance and facet preservation than can be achieved via the IL approach. Larger-scale studies are needed for better delineation and for correlation of radiological features with clinical manifestations.
Introduction
Lumbar spinal stenosis is the pathology seen most often in spinal surgery patients aged 65 or older [1]. The definition includes two entities: (1) clinical manifestations and (2) radiological morphological abnormalities seen in imaging [2]. Clinical manifestations can present as different varieties, from initial positional radiculopathy, to the more classical neurogenic claudication, to the severe form of cauda equina syndrome [3]. Radiological stenosis in the central canal arises commonly from concomitant ligamentous hypertrophy and disk protrusion, particularly in pre-existing congenital small spinal canals.
Surgical decompression is considered for concordant pain and/or neurological symptoms that do not respond to conservative therapy. Though the success rate of open decompression is variable, ranging from 64% to 69% [1], extensive disruption of bony and soft tissue can have adverse consequences, including flexion instability, muscle weakness, and failed back syndrome [4]. Minimally invasive spinal surgery aims to decompress the symptomatic neural elements without violating spinal stability. Over the years, surgical techniques have evolved from mini-open to microscopic, micro-endoscopic, and endoscopic surgery.
For spinal stenosis presenting with unilateral clinical manifestations and corresponding radiological stenosis, decompression via the open ipsilateral (IL) approach appears to be a more direct and shorter route for decompression that has been reported to be effective and has been popularized traditionally [5]. Bilateral decompression using full-endoscopic [6] and biportal endoscopic [7] methods for spinal stenosis has also been described. More recent literature has suggested that endoscopic decompression may be safer and more easily accessible from the contralateral (CL) side of pathology [8]. However, no study has yet published the radiological outcome of comparison of IL versus CL endoscopic decompression.
The aim of this study is to examine the immediate postoperative radiological outcomes of IL versus CL biportal endoscopic decompression for unilateral spinal stenosis.
Materials and Methods
1. Study design and patients
From May to October 2019, we retrospectively reviewed patient demographics, operative records, and radiological magnetic resonance imaging (MRI) imaging of patients with unilateral biportal endoscopic spinal decompression surgeries performed in Daejeon Woori Hospital, Korea. In addition, we analyzed pre- and postoperative numerical Visual Analog Scale (VAS) pain scores for the lower limb during in-hospital stay. We calculated the percentage of VAS pain reduction as preoperative VAS minus postoperative VAS/preoperative VAS×100.
We obtained the institutional review board approval from the Research Ethics Committee, Kowloon West Cluster, Hong Kong (Ref: KW/EX-21-025 [156-07]). Informed Consent was waived.
This study included patients who fulfilled all of the following inclusion criteria: (1) predominant unilateral lower limb neurogenic claudication or neurological symptoms, (2) compatible radiological MRI imaging of degenerative spinal stenosis on the same side as and the same level of clinical manifestation due to flavum hypertrophy either with or without disk bulging, and (3) immediate postoperative MRI imaging on the same day as surgery. Exclusion criteria included (1) either significant motion artifacts or poor imaging quality; (2) multi-level stenosis of three or more levels; (3) significant disk prolapse either posterior to the intra-facet line or with cranial/caudal migration; (4) space occupying lesions in the spinal canal, e.g., facetal cysts; (5) predominant foraminal or extra-foraminal stenosis; (6) pathologies other than degenerative conditions, e.g., malignancy or spondylolisthesis requiring stabilization; and (7) prior history of spinal operations.
2. Surgical procedure
Before surgery, MRI images of patients undergoing spinal operation were evaluated, and the surgeon responsible carried out operative templating of the approach trajectory and the designated approach to determine whether to use an IL or a CL approach. There was no randomization of the surgical approach.
Biportal endoscopic spinal decompression of the stenotic level was carried out by the same two experienced spinal surgeons in the center. Both surgeons are right-handed. Following either general or spinal anesthesia, the patient was placed in a prone position, and fluoroscopic imaging was used to confirm the operative level. Two small incisions were made 5 mm lateral to the spinous process, followed by insertion of endoscopic portals with gravity water inflow. The muscle layer was separated from the lamina bone by blunt dissection and cauterization. The spinolaminar junction was identified. Unilateral laminotomy was conducted using a bone burr, following a pre-designated surgical approach. The midline cleft was identified. Flavectomy was carried out with dural decompression on the symptomatic side until the pedicle, thecal sac, and facet margin were reached. For facet hypertrophy obscuring exposure of flavum insertion, partial undercutting of the ventromedial facet was carried out. Undercutting of the superior articular process was carried out for lateral recess stenosis with nerve root impingement. Disk sequestrectomy was carried out whenever neural elements remained impinged by the bulging disk after flavectomy and bony undercutting. Partial resection of the flavum of the asymptomatic side was performed until adequate decompression of the dural sac was achieved. Following decompression, all patients received 5 mL Floseal and low-suction drains (Baxter Healthcare Corp., Hayward, CA, USA).
3. MRI imaging evaluation (Fig. 1)
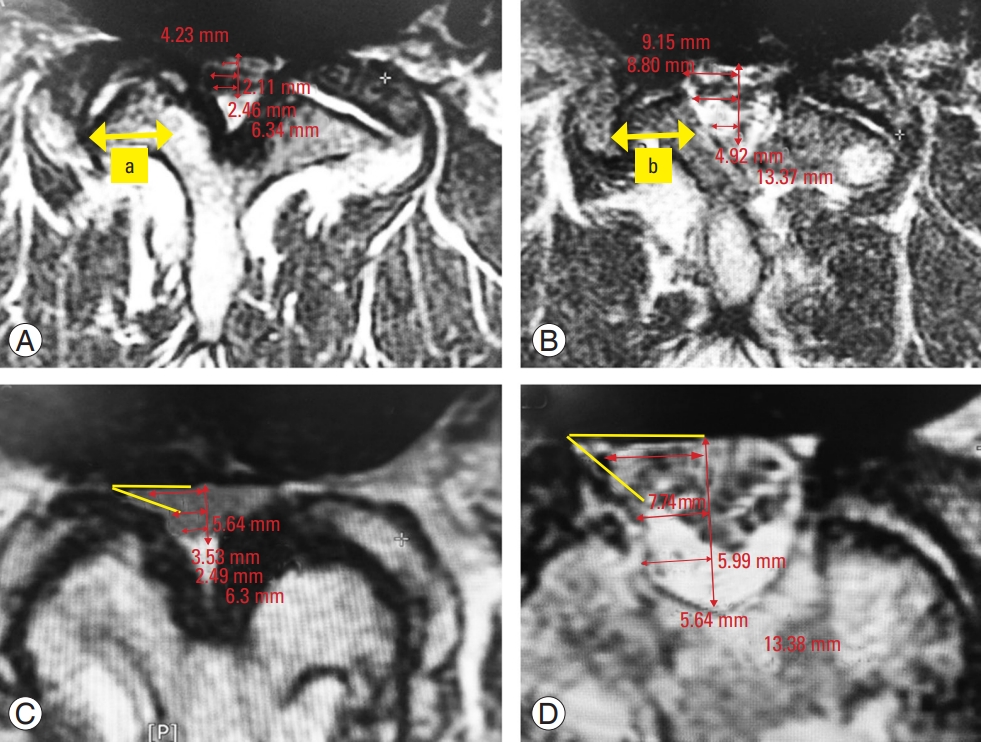
Measurement method of radiological parameters in axial magnetic resonance imaging. (A) Measurement of facet length before surgery. (B) Facet length after surgery. The percentage of facet preservation is calculated by the ratio of postoperative over preoperative parameters, i.e., facet preservation (%)=(b/a×100). (C) Measurement of recess angle before surgery. (D) Improvement of recess angle and recess height after surgery. AP, anteroposterior.
Electronic pre- and postoperative MRI imaging was carried out using the STARPACS, π-ViewSTAR 5.0.9.101 (INFINITT MRI imaging system; INFINITT Healthcare Co. Ltd., Seoul, Korea). The transverse T2-weighted MRI images of the most stenotic level were adopted for measurement. The mid-sagittal reference line was drawn through the center of the vertebral body or disk and the center of the spinolaminar junction [9]. This line is used as reference to ascertain the radiological diameters of the hemi-dural sac, which reflects the radiological severity of unilateral spinal stenosis.
We defined the percentage of facet preservation as the length of the postoperative facet/the length of the preoperative facet×100 [10] (Fig. 1A, B). We defined the lateral recess angle as the angle between the floor of the lateral recess and the ligament flavum on the ventral side of the inferior articular process [2] (Fig. 1C, D). We measured the height of the lateral recess between the most anteromedial portion of the superior articular facet and the posterior surface of the vertebral body [2]. We measured the recess diameter of the dural sac from the most anterolateral sac border to the mid-sagittal line.
4. Statistical analyses
We calculated the dural sac expansion ratio as the ratio between pre- and postoperative parameters. We carried out statistical analyses using IBM SPSS ver. 26.0 for Windows (IBM Corp., Armonk, NY, USA). Data were entered by a research assistant who was blind to patient grouping. For calculating p-values, we used a two-tailed t-test for continuous variables and Fisher’s test for categorical variables. A p-value lower than 0.05 was considered statistically significant.
Results
Between May to October 2019, we enrolled 28 male and 33 female patients who fulfilled the inclusion and exclusion criteria. Four male and six female patients received two-level spinal decompression surgeries. Unilateral biportal surgery was performed using the IL approach in 37 spinal segments and the CL approach in 34 segments. All but one of the IL approaches were carried out on the patient’s left-hand side. For the CL approach, 22 were carried out on the patient’s left-hand side and 12 were carried out on the right-hand side. Operations were carried out at three L2/3 levels, 16 L3/4 levels, 45 L4/5 levels, and seven L5/S1 levels. There was no statistical difference in respect of operative times (69.0 versus 69.2 minutes, p=0.983). We did not observe any statistical differences between the IL and CL approaches in respect of age, gender, and preoperative radiological parameters (Table 1).
In the IL and CL groups, we observed statistical differences in the postoperative recess angle (Fig. 2), the recess height (Fig. 3), and the dural sac expansion ratio for recess diameter (Fig. 4), with the CL group displaying better lateral recess decompression. The percentage of facet preservation in the IL group was significantly lower than was that in the CL group (83.7% versus 91.9%, p=0.001). No in-hospital mortality was reported. One patient who had CL approach surgery complained of persistent right leg pain and received a second operation using the IL approach 6 weeks later. The symptoms resolved after the second operation. There were neither any wound complications nor other postoperative morbidities.

Recess angle before and after operation in ipsilateral (IL; blue) and contralateral (CL; green) approach groups. Values are represented as mean±95% confidence interval. Statistical significance was evident after operation.
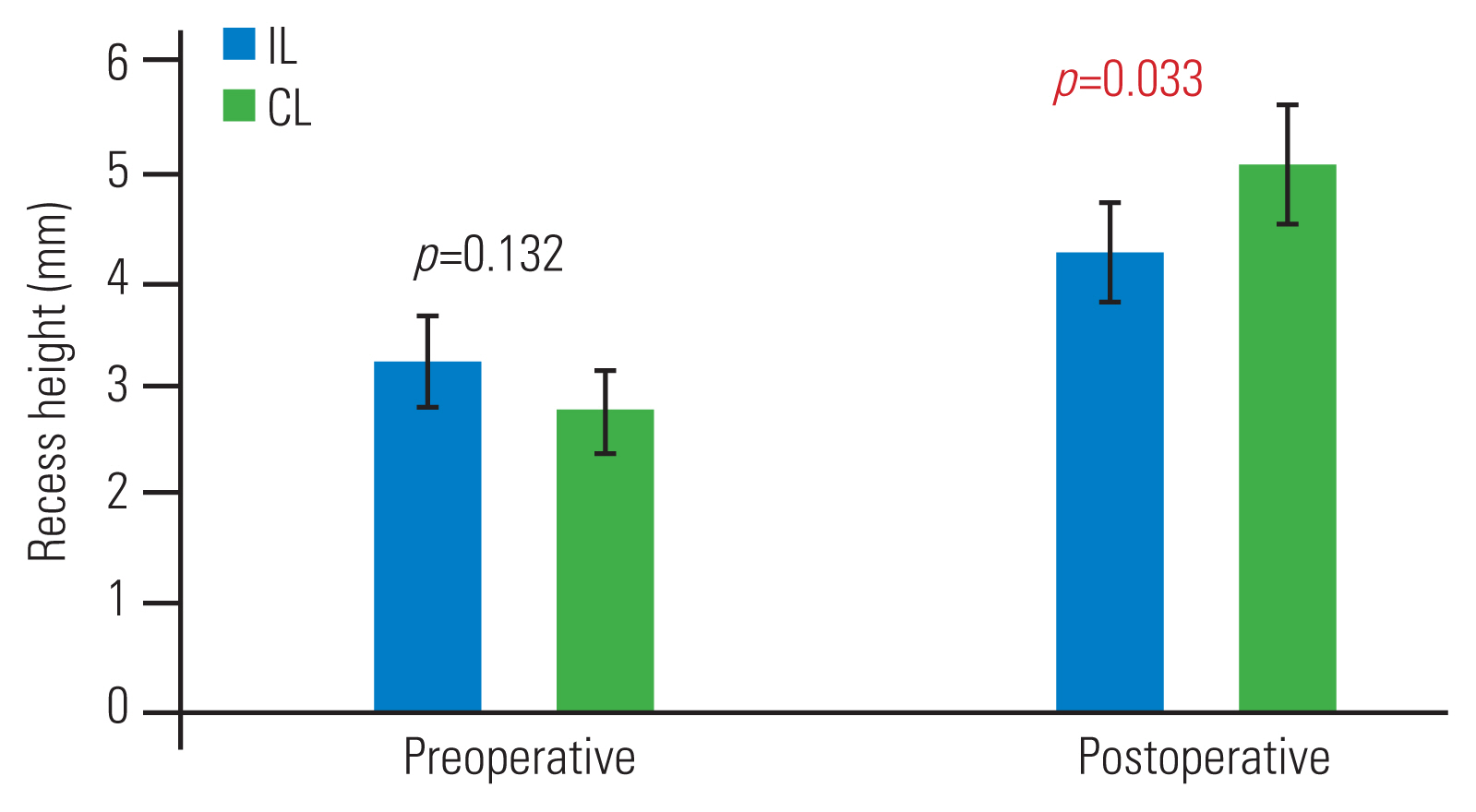
Recess height before and after operation in ipsilateral (IL; blue) and contralateral (CL; green) approach groups. Values are represented as mean±95% confidence interval. Statistical significance was evident after operation.
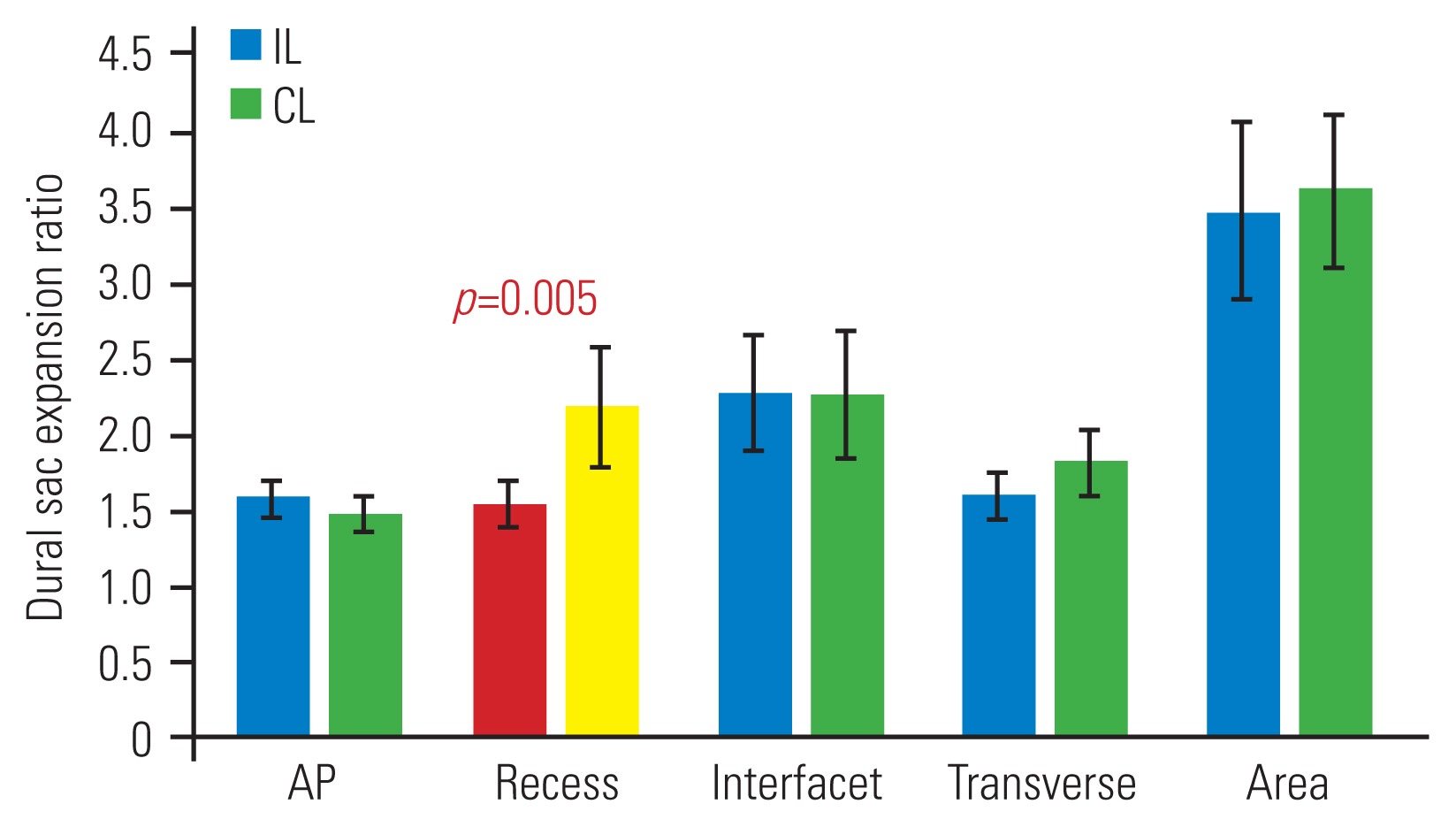
Postoperative dural sac expansion ratio. Values are represented as mean±95% confidence interval. IL, ipsilateral; CL, contralateral; AP, anteroposterior.
We carried out subgroup evaluation of the left-sided IL versus the left-sided or right-sided CL approaches. In both the left-sided and the right-sided CL subgroups, there was significant improvement of lateral recess clearance in respect of recess angle (Fig. 5), recess height (Fig. 6), and dural sac expansion ratio for recess diameter (Fig. 7).
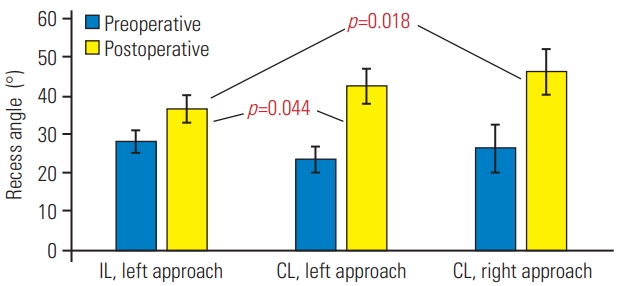
Subgroup analysis of recess angle before and after operation. Values are represented as mean±95% confidence interval. Preoperatively, no statistical significance was observed. Postoperatively, statistical significance was evident in the ipsilateral left versus contralateral left/right groups. IL, ipsilateral; CL, contralateral.
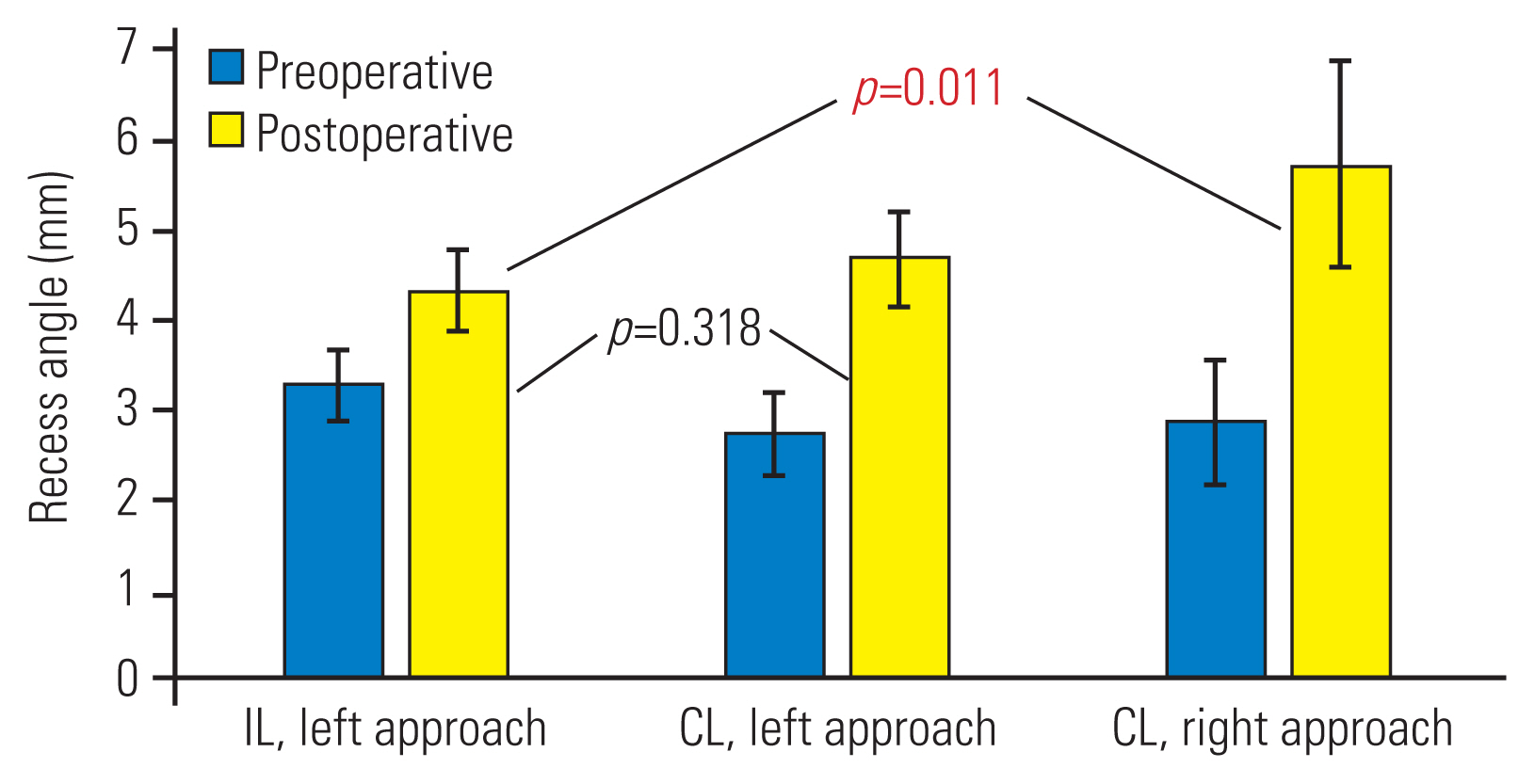
Subgroup analysis of recess height before and after operation. Values are represented as mean±95% confidence interval. Preoperatively, no statistical significance was observed. Postoperatively, statistical significance was evident in the ipsilateral left versus contralateral right group. IL, ipsilateral; CL, contralateral.
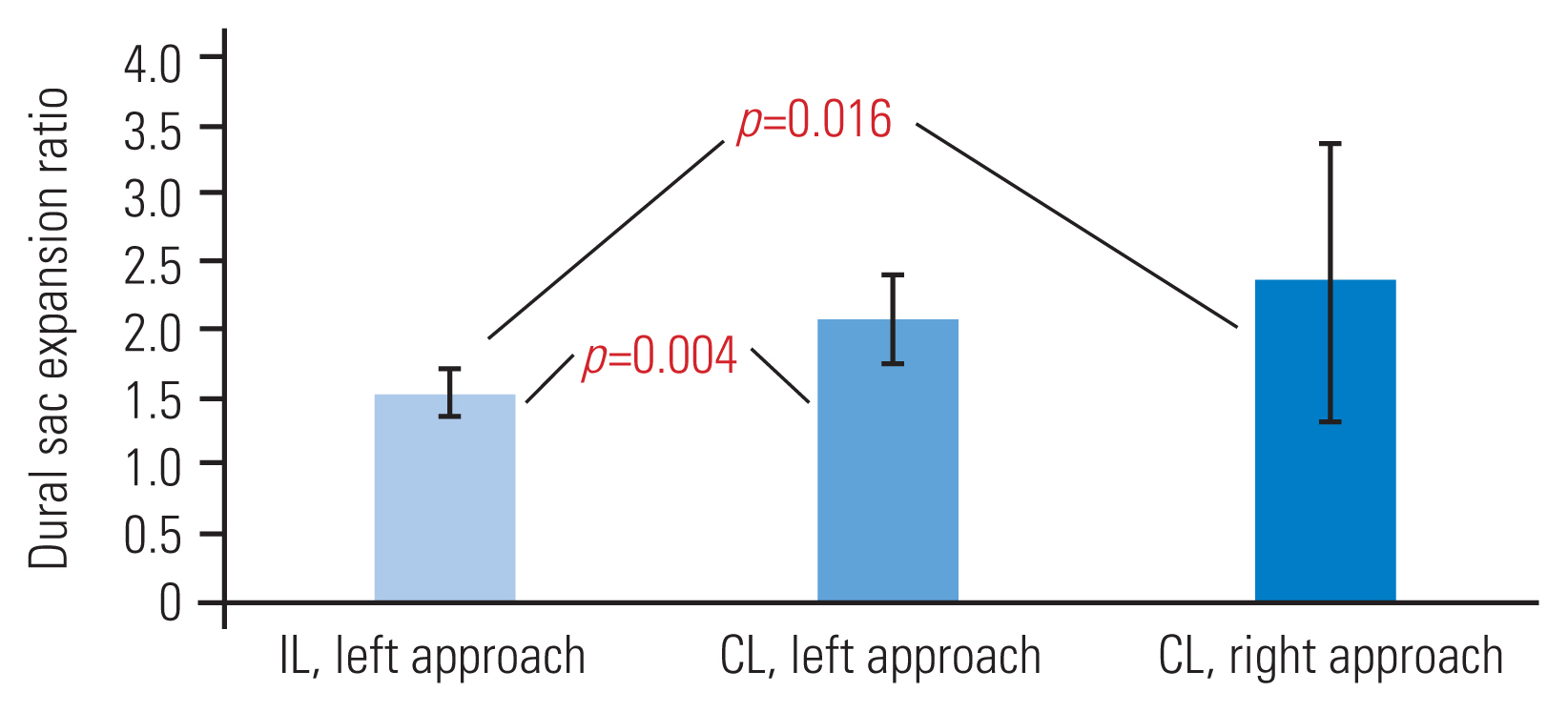
Subgroup analysis of dural sac expansion ratio (recess diameter). Statistical significance was evident in the ipsilateral left versus contralateral left/right groups. IL, ipsilateral; CL, contralateral.
VAS in the IL and CL groups improved by 69.3% and 63.6%, respectively. We did not find any statistically significance difference in respect of VAS reduction and percentage improvement between the IL and CL approaches (Fig. 8).
Discussion
The most common type of spinal stenosis is caused by degenerative changes of the disk, facet joints, and ligamentum flavum. Dural tube stenosis arising from changes in the three-joint complex, including the disc and the facet joints, is morphologically described as “trigonal compression.” The lateral recess is narrowed by osteophytes over the superior articular process, and the central canal is narrowed by overgrowth of the inferior articular process. Symptoms of lateral stenosis generally involve specific dermatomes due to root compression, whereas central stenosis presents more commonly with neurogenic claudication, including both the buttocks and posterior thighs [11]. Both lateral and central stenosis are linked closely in the genesis of symptoms in patients with marked degenerative changes, and they often coexist [12,13].
Though neurogenic claudication is the most common presenting symptom in spinal stenosis, the literature shows that more than half of spinal stenosis cases have presented with unilateral radiculopathy and that only 42% to 43% have been associated with bilateral radiculopathy of the lower extremities [14,15]. Open surgeries that involve extensive disruption of bony and muscular structures induce a large dead space for colonization by bacteria and formation of perineural scars, resulting in chronic pain and failed back surgery syndrome [16]. Bilateral open laminectomy is more invasive and may cause instability of the lumbar spine, and the extensive surgical dissection involved may aggravate blood loss and increase the possibility of dural rupture or other complications that could exacerbate the pre-existing radiculopathy [17].
There is a trend toward limiting midline and CL resection while not compromising neural decompression [16]. A unilateral laminotomy series concluded no constant correlation between clinical symptoms and the severity of radiological stenosis. Laminotomy was carried out only on the symptomatic side, irrespective of radiological findings on the asymptomatic side [5].
Given that a significant proportion of patients present only with unilateral radiculopathy, it is hypothesized that it may not be necessary to decompress the asymptomatic side [18]. In series of Zhang et al. [18], the lamina, facet joint, and flavum of the asymptomatic side were left intact. Decompression up to the midline was carried out on the symptomatic side until the base of spinous process was reached [18]. In our series, the symptomatic side was first decompressed after unilateral laminotomy. The lamina on the asymptomatic side was left mainly intact, with only partial flavectomy being carried out to decompress any residual central stenosis.
The literature contains little radiological definition of lateral recess stenosis. The use of lateral recess angle and height has been mentioned [2,19]. In our study, the recess diameter of the dural sac also serves as a simple and convenient way to represent the severity of traversing root compression when the classical “trefoil” appearance is distorted at the lateral recess.
Series of Park et al. [5] of 39 patients with microscopic unilateral laminotomy reported a radiological outcome of 187% IL dural sac widening and 146% CL widening 1-month postoperation, showing statistical significance between the two groups. Conversely, our series found better improvement in recess diameter with the CL approach than with the IL approach (217% versus 154%, p=0.005). Although radiological decompression outcomes differ significantly, both studies found similar clinical outcome in both groups.
Besides adequacy of lateral recess decompression, another important purpose of adopting CL rather than IL decompression is minimization of violation of the facet joint. Matsumura et al. [10] reported 80% and 95% facet preservation in IL and CL miscroscopic spinal decompression, respectively. We have achieved a similar result, with 84% versus 92% preservation in biportal endoscopic spinal decompression. The CL approach, which adopts a more oblique trajectory, follows the anatomical configuration of the oblique recess roof. In IL spinal surgery, a greater portion of the facet joint must be resected before the lateral recess can be visualized readily.
Although there is no proof in the existing literature, we believe that hand dominance and laterality of the pathology may be related to the technical difficulty of carrying out biportal endoscopic spinal surgery. Bone work begins typically from the interlaminar space and moves toward the more cranial lamina bone, with the dominant hand holding the working instruments. Thus, a right-handed surgeon may find it easier to operate easier using the left-sided-approach, whereas a left-handed surgeon may prefer the right-sided approach. Although we demonstrated better lateral recess decompression in both left- and right-sided CL than in left-sided IL, statistics from a right-sided IL approach were lacking. This is one of the limitations of our study.
Our study has other limitations. There are no longer-term radiological results to delineate dural sac expansion, as most patients had been lost to follow-up once their symptoms resolved. Immediately postoperation, the dural sac expansion space may be occupied by drains, hematoma, and irrigation fluid. There is a lack of specialized computer programs for precise measurement of dural sac parameters. Measurement of spinal stenosis is confined by two-dimension, rather than three-dimension, stereoscopic and dynamic imaging. The existing literature has focused on bony canal measurements, which are of limited comparison value to our study of dural sac expansion.
Conclusions
Unilateral biportal interlaminar decompression by the CL approach may offer better lateral recess clearance than does the IL approach, with the added advantage of facet preservation. Larger-scale studies are needed for better delineation and for correlating radiological features with clinical manifestations.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.