Single-Stage Posterior Approach for the En Bloc Resection and Spinal Reconstruction of T4 Pancoast Tumors Invading the Spine
Article information
Abstract
Study Design
Retrospective cohort study.
Purpose
This study aimed to evaluate the outcomes of patients who had T4 Pancoast tumors invading the spine and underwent en bloc resection and spinal stabilization through a single-stage posterior approach.
Overview of Literature
Surgical resection for Pancoast tumors affecting the spine has been successfully performed in two stages involving spinal reconstruction and tumor resection. However, reports have rarely presented the results of en bloc resection combined with spinal stabilization for T4 Pancoast tumors invading the spine through a single-stage posterior approach.
Methods
Patients who had T4N0M0 Pancoast tumors invading the spine and underwent a single-stage posterior approach were retrospectively recruited. The following data were obtained and examined: demographics, tumor histology, preoperative and postoperative therapy, complications, spinal reconstruction technique, tumor resection extent, survival time, and disease recurrence.
Results
Eighteen patients were included. The mean population age was 61±17 years, and the most common pathological type was adenocarcinoma (61.1%). Complete resection (R0) was obtained in 15 patients (83.3%), positive surgical margins (R1) were found in three patients (16.7%), and the 90-day mortality rate was 0%. Postoperative major complications were detected in 12 patients (66.7%), who required reoperation. The mean survival time was 67±24 months, but the median survival time was not reached. Among the patients, 10 (55.6%) are still alive at the end of the study. The 2- and 5-year actual survival rates were 59% (95% confidence interval [CI], 35.7%–82.3%) and 52.5% (95% CI, 28.4%–76.6%), respectively.
Conclusions
En bloc resection and spinal stabilization through a single-stage posterior approach might be effective for T4 Pancoast tumors invading the spine.
Introduction
Superior sulcus tumors or Pancoast tumors, which were named after Henri Pancoast, who first described them in 1924 [1], are relatively rare neoplasms accounting for <5% of all non-small-cell lung carcinomas [2]. These tumors are difficult to manage; initially, they are subjected to palliative treatment because of the tumoral involvement of adjacent critical structures, such as the chest wall, subclavian vessels, brachial plexus, and spine [3]. In 1953, the first case of a long-term survival of a patient with Pancoast tumor following surgical removal and postoperative radiation therapy was reported [4]. These tumors have been treated with several surgical approaches, such as Shaw-Paulson posterior thoracotomy [5]; anterior approach performed by Dartevelle and Mentzer [6], subsequently modified by Grunenwald and Spaggiari [7]; hemiclamshell thoracotomy with suprasternal (trapdoor) extension [6,8,9]; and approaches with lung resections through minimally invasive techniques [10–12].
In the past, surgical resection for Pancoast tumors involving the spine was conventionally contraindicated because of the expected poor outcomes. With advancements in spinal reconstruction techniques, some surgeons successfully performed spinal reconstruction through a posterior approach (first stage) and subsequent en bloc resection through Shaw–Paulson or trapdoor thoracotomy (second stage) for this type of tumor [13–15]. In 2008, Jain et al. [16] described a single-stage posterior approach for en bloc resection combined with spinal stabilization in two patients with Pancoast tumors. They suggested that this technique may reduce postoperative recovery time and minimize surgical trauma, surgery time, and related complications. However, reports about the results of this surgical strategy with a larger series, especially T4 Pancoast tumors with spinal involvement, are limited [16,17].
In this study, patients who had T4 Pancoast tumors invading the spine and underwent en bloc resection and spinal stabilization through a single-stage posterior approach were retrospectively analyzed to determine whether this surgical approach was safe and effective for treating these tumors.
Materials and Methods
1. Patient selection
All patients who had T4N0M0 Pancoast tumors invading the spine and underwent a single-stage posterior approach at Centre Hospitalier de l’Université de Montréal (CHUM) from January 1, 2014, to August 31, 2020, were included in this study. Pancoast tumors were diagnosed according to the established clinical and radiological criteria and classified according to the eighth edition of the tumor-node-metastasis staging for non-small-cell lung cancer developed by the International Association for the Study of Lung Cancer [18]. This study was approved by the research ethics committee of Research Center, Centre Hospitalier de l’Université de Montréal (CHUM) (approval no., 20.271).
Physical examination and chest computed tomography (CT) scan were performed for all patients. Magnetic resonance imaging with gadolinium was performed to evaluate the tumoral invasion of the spine, brachial plexus, and major vessels. Positron emission tomography scan was used to rule out distant metastasis and evaluate the involvement of mediastinal lymph nodes. Invasive mediastinal staging was conducted in all cases via an endosonography-guided approach with a combination of endobronchial ultrasound-guided transbronchial needle aspiration and endoscopic ultrasound-guided fine needle aspiration. Patients with histologically proven mediastinal node involvement or distant metastasis were excluded because they were ineligible for curative-intent surgery. Patients considered to have poor cardiopulmonary performance and medically unfit for surgical resection were also excluded. Informed consent was obtained from all included patients.
2. Treatment planning
Patients who were subjected to an induction treatment with two concomitant cycles of chemotherapy (cisplatin–VP16) and radiotherapy underwent surgery 4–6 weeks after their completion. The vertebral involvement of the tumors was preoperatively classified into types A, B, and C to determine the extent of vertebral body resection and spinal reconstruction (Fig. 1).
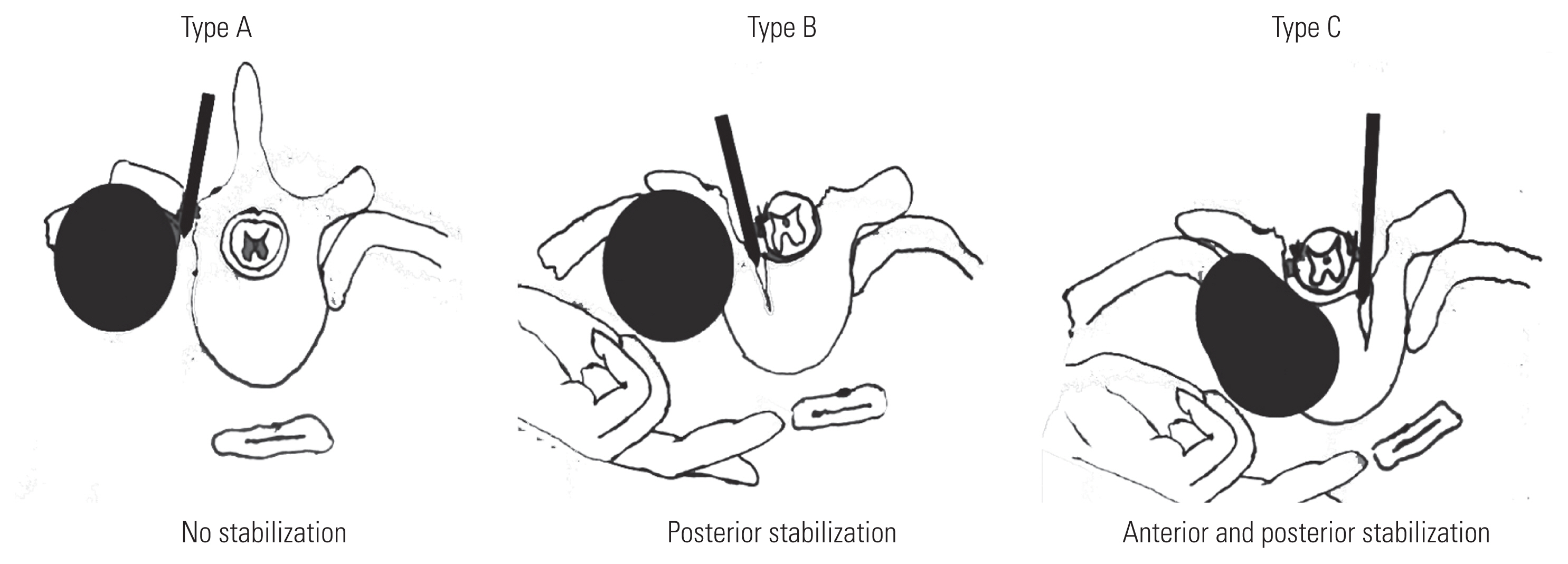
(A–C) Artist’s illustration of extent of vertebral body resection and spinal stabilization according to the type of spinal involvement. Adapted from Zairi F, et al. Asian Spine J 2016;10:1047–57
The patients were evaluated by an oncologist who determined whether they were fit enough to receive adjuvant therapy after surgery.
3. Surgical technique
All patients were subjected to curative-intent surgery comprising en bloc resection of the upper lobe of the lung and wedge resection of the adjacent lobe as needed, chest wall, and vertebrae. The surgical technique was described in detail in a previous study [17]. A patient is placed in a prone position on a Jackson table, and the head is secured in a three-pin Mayfield headrest. A posterior midline incision is made along the spinous processes between C5 and T10. Laminae, facets, and transverse processes are exposed. If vertebrectomies are planned, screws are placed in the C5 and C6 lateral mass, C7 pedicles (Vertex; Medtronic, Minneapolis, MN, USA), and thoracic pedicles in the uninvolved side of the affected vertebrae (Legacy; Medtronic). Thoracic pedicle screws are inserted in both sides from three to five levels below the most caudal affected vertebra, depending on the number of vertebrectomies required. Then, laminectomies at the affected vertebrae are performed, and their nerve roots are clipped and cut on the ipsilateral side in the case of partial vertebrectomies and bilaterally in the case of total vertebrectomies. The T1 nerve root may be cut if necessary, but the C8 nerve root must be preserved. For patients who require total vertebrectomies, discectomies are performed at the proximal and distal extremities of the affected levels to allow their further mobilization. A transitional rod with a transition from a diameter of 3.5 mm at the cervical level to a diameter of 5.5 mm at the thoracic level is placed on the uninvolved side to ensure spinal stability.
Subsequently, a thoracic surgery team performs a posterior incision extended laterally up to the posterior axillary line and connects the upper and posterior extremity to the posterior midline incision performed previously by a spine surgeon (Fig. 2). After the trapezius, rhomboid muscles, and intercostal plane are transected, the pleural cavity is opened, and the rib retractor is placed. The chest cavity entry site is planned to be at least 4–5 cm lateral and inferior to the tumor. The posterior segment of the involved ribs is resected, and their neurovascular bundles are ligated. The scapula is lifted away from the thoracic inlet to control the subclavian vessels more effectively and visualize the lower trunk of the brachial plexus adequately. Then, upper lobectomy is performed through thoracotomy. Although lateral flexion is applied to a surgical table, the posterior approach on the patient in the prone position may pose an additional challenge to surgeons. Hilar dissection is initially performed in the posterior aspect and directed anteriorly as structures are ligated. Usually, the bronchus is ligated early, followed by the pulmonary artery (PA) branches and the pulmonary vein. On the left side, according to surgeons’ preference, the PA posterior ascending and apical branches can be ligated before the bronchus. Lung resection with a fissure-less approach is routinely performed to reduce postoperative air leakage. Vascular and bronchial structures, as well as fissures, are transected via standard techniques by using surgical linear staplers (Echelon Flex; Ethicon Inc., Somerville, NJ, USA).
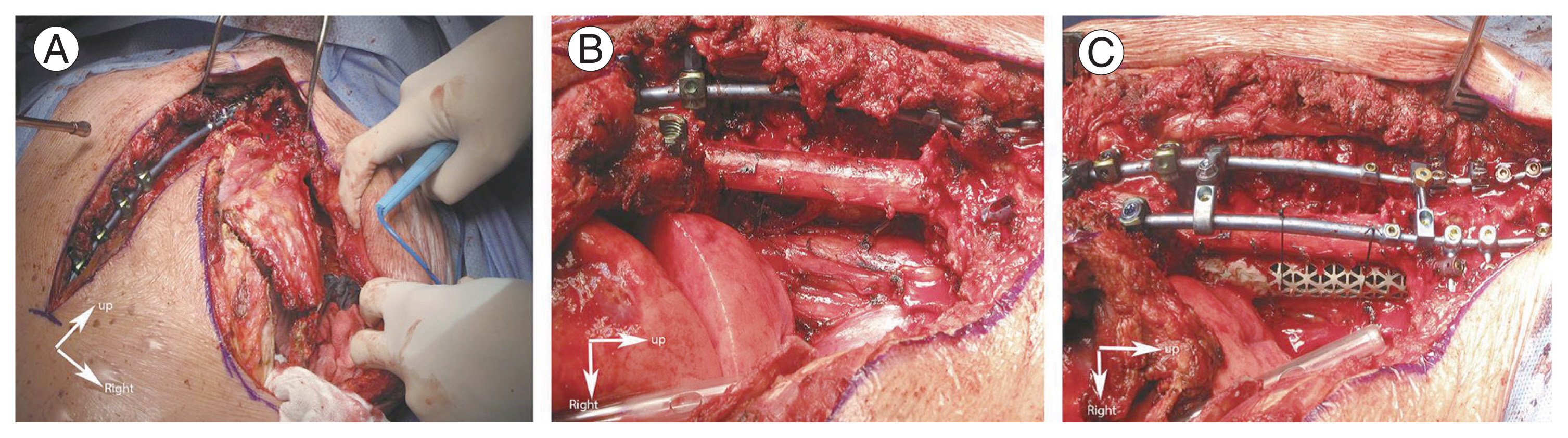
Intraoperative photograph. (A) Posterior spinal stabilization is performed first, then a lateral incision is used to expose the chest wall. (B) En bloc resection of the tumor is carried out. (C) PYRAMESH (Medtronic Sofamor Danek USA Inc., Memphis, TN, USA) is used to complete the anterior spinal reconstruction. Adapted from Zairi F, et al. Asian Spine J 2016;10:1047–57 [17].
Mediastinal structures, notably the aorta and esophagus, are sequentially separated from the anterior edge of vertebral bodies through blunt finger dissection. Vertebrectomies are then performed using osteotomes at all levels. A spine surgeon determines the amount of vertebrectomy in terms of the extent of tumor invasion into the vertebral body, which is preoperatively classified into types A, B, and C. For type A tumors, no vertebrectomy is performed. For type B tumors, vertebrectomies are extended to the medial border of the ipsilateral pedicle. For type C tumors, vertebrectomies are extended to the medial border of the contralateral pedicle. The spine surgeon continues muscle and ligament dissection to remove the tumor en bloc. Afterward, a thoracic surgeon performs complete mediastinal lymphadenectomy. Spinal stabilization is completed by placing and locking the second transitional rod on the involved side. In patients who undergo total vertebrectomies, a pyramesh cage (PYRAMESH; Medtronic Sofamor Danek USA Inc., Memphis, TN, USA) is inserted for anterior spinal construction (Fig. 2). At the end of the procedure, a posteriorly positioned chest tube is placed, and the chest wall is closed with the scapula to protect the posterior defect as much as possible.
4. Data collection
The following data were collected: recurrence information, demographics (age and gender), tumor histology, tumor staging, preoperative and postoperative therapy, perioperative data, spinal reconstruction types, tumor resection extent, margin status, and postoperative survival time, which was defined as the time between the date of surgery and the date of death or the most recent follow-up. Distant recurrence and metastasis were differentiated. Distant recurrence was defined as the presence of a new lung cancer tumor outside the surgical field, and metastasis was defined as the presence of extrapulmonary tumoral sites. Postoperative morbidity was evaluated according to the Common Terminology Criteria for Adverse Events version 5.0 [19].
5. Statistical analysis
The Kaplan-Meier method was applied to calculate postoperative survival time. Statistical analysis was performed using IBM SPSS ver. 21.0.0 (IBM Corp., Armonk, NY, USA).
Results
1. Overall
During the study period, two patients who had T4N0M0 Pancoast tumors invading the spine could not undergo surgery because of poor cardiopulmonary status, and eighteen patients who had T4N0M0 Pancoast tumors with vertebral involvement and underwent surgery were analyzed. The mean population age was 61±17 years (range, 50–75 years), the male/female ratio was 1.57, the mean number of vertebrae invaded by the tumors was 3.2±1.2 (range, 2–4), and the most common pathological type was adenocarcinoma (61.1%). The characteristics of the study population are summarized in Table 1.
2. Treatment and outcome
In this cohort of 18 patients, 16 completed the induction treatment comprising concomitant chemotherapy (two cycles of cisplatin–VP16) and radiation therapy (delivered radiation dose of 45–50 Gy). Among them, 14 (87.5%) responded to the treatment.
Two patients underwent surgery without induction treatment because of severe mechanical pain and overt instability of the CT. Two patients with a type C tumor underwent spondylectomy combined with anterior and posterior spinal construction, twelve patients with a type B tumor had hemivertebrectomy and posterior spinal stabilization, and four patients with a type A tumor had partial corpectomy but did not require spinal fusion. One patient required a bypass of the brachiocephalic and subclavian arteries during tumor resection and had blood loss of 14,000 mL. The mean surgical time was 446±85 minutes (range, 160–701 minutes), the mean surgical blood loss was 2,947±1,494 mL (range, 650–14,000 mL), and the mean in-hospital length of stay was 44±18 days (range, 10–119 days).
Complete resection (R0) was obtained in 15 patients (83.3%), and positive surgical margins (R1) were found microscopically on the final pathological specimen in three patients (16.7%). The perioperative data and treatment outcomes are summarized in Table 2. Furthermore, four patients received adjuvant chemotherapy, but 14 patients did not undergo this treatment because of their unfavorable recovery, fragility, or refusal. Another patient had immunotherapy in addition to adjuvant chemotherapy.
The 90-day mortality rate was 0%. Twelve patients (66.7%) who had major postoperative (CTCAE [Common Terminology Criteria for Adverse Events] grade 3/4) complications, including wound complication, cerebrospinal fluid leakage, chylothorax, hemothorax, respiratory insufficiency, or rod fracture, had to return to the operating room. Pneumonia was the most common complication (38.9%). One patient presented with left arm paralysis after surgery because the brachial plexus was sacrificed during tumor resection. Seven cases (38.9%) had wound dehiscence, and five patients (27.8%) had surgical site infection. The details of complications and management are described in Table 3.
3. Survival and tumor control
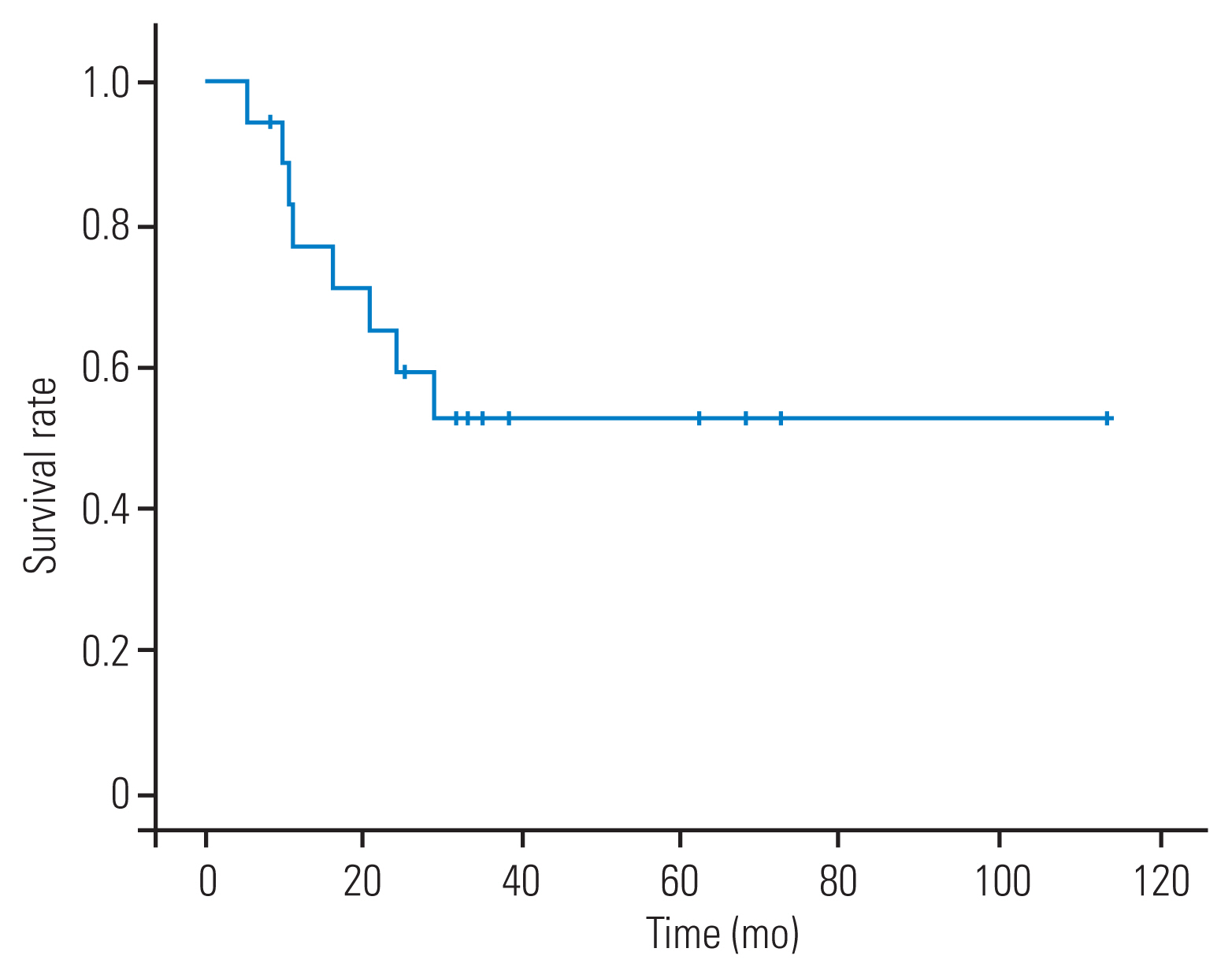
The mean follow-up time was 27±10 months (range, 4–74 months). The mean postoperative survival time was 67±24 months (range, 5–114 months), but the median postoperative survival time was not reached. The median follow-up time of 10 patients (55.6%) still alive at the end of the study was 29 months (range, 4–74 months). The actual 2- and 5-year survival were 59% (95% confidence interval [CI], 35.7%–82.3%) and 52.5% (95% CI, 28.4%–76.6%), respectively (Fig. 3). Four patients (22.2%), namely, one with R1 resection and three with R0 resection, developed local recurrence after surgery. Three patients (16.7%) had distant recurrence.
Discussion
Different surgical approaches for successful resection of locally advanced Pancoast tumors with multilevel vertebral involvement have been reported [15,17,20,21]. In cases requiring spinal stabilization, these techniques are typically composed of spinal stabilization in the first stage and tumoral resection in the second stage. In this study, the efficacy and safety of en bloc resection and spinal stabilization through a single-stage posterior approach in patients having T4 Pancoast tumors with spinal involvement were assessed.
1. Surgical outcomes and complications
The mean operative time in our series was 446±85 minutes, which was similar to that reported by Jain et al. [16]. The average blood loss in our series was 2,947±1,494 mL, which was higher (675 mL) than that in the two cases described by Jain et al. [16]. The mean length of hospitalization of our patients was 44±18 days, which was longer than that (9–11 days) reported in previous studies [16,22,23].
Previous studies showed that the surgical treatment of locally advanced Pancoast tumors is a highly complex procedure with a high complication rate, which ranges from 28% to 53.2% [14,22–27]. The major complication rates (66.7%) in our series were relatively higher than those in previous studies. In our series, 12 patients (66.7%) required reoperation, and this number was higher than that in the series of Gandhi et al. [22] (5.8%) and Solli et al. [23] (9.3%). Wound complication was the main cause of reoperation (six of 12 patients). Some reasons might explain the higher complication and reoperation rates in our series. Among the causes of discrepancies in morbidity in different studies are variations in tumor stages and comorbidities. A potentially more complex surgery performed in our series might be another reason. For example, all the patients in this study had pathological stage T4N0M0, and 14 (77.8%) required vertebrectomy and spinal reconstruction. In the series of 88 patients described by Rusch et al. [24], only 11 patients (13.2%) with T4 Pancoast tumors underwent vertebrectomy. In another series of 57 patients reported by Kunitoh et al. [28], 11 patients (19.2%) with T4 Pancoast tumors had thoracotomy, but information about vertebral resection and spinal reconstruction was not provided. Similarly, Waseda et al. [27] described 28 patients who had T4 Pancoast tumors and underwent surgery, but only 11 (39.2%) had spinal procedures. Preoperative radiation dose might be a risk factor for wound issues. Of the 18 patients in this study, 16 received induction chemoradiation with a dose of 45–50 Gy. The delivered radiation dose was greater than 35 Gy used in the studies of Gandhi et al. [22] and Alifano et al. [26] and equivalent to 45 Gy reported in other series [24,28]. Some surgeons favored surgery as the first step in treating patients with Pancoast tumors because of the high rate of chemotherapy- or radiotherapy-induced complications [23,26,29]. Solli et al. [23] found that this approach does not compromise the overall rate of complete resection. Yildizeli et al. [29] reported that induction chemotherapy unlikely increases the completeness of resection; however, their population studies involved Pancoast tumors with different stages. Considering the complexity of T4 Pancoast tumors invading the spine, we are unsure if their approaches are appropriate to achieve complete resection in this subset of Pancoast tumors. An advantage of induction chemoradiotherapy is facilitating complete resection by shrinking the tumor before surgery [24,28,30]. The R0 resection rate in our study was 83.3%, which was favorably comparable with the results in previous reports (56%–96%) [14,20,22,23,26,27]. Patients in previous studies had Pancoast tumors with different stages and underwent different surgical approaches, whereas all patients in our series had pathological T4 Pancoast tumors invading the spine. Moreover, induction chemoradiotherapy significantly affects the survival of patients with Pancoast tumors [24,28,30]. Solli et al. [23] observed an excellent 5-year survival (85%) in seven patients with a complete response after induction treatment; therefore, induction chemoradiotherapy is an effective strategy for treating T4 Pancoast tumors invading the spine. To minimize wound complications, a plastic surgeon should participate in surgical planning and wound closing.
Previously reported posterior-combined approaches might pose anatomical challenges to thoracic surgeons because they are not used to performing lung resections with a patient positioned in a ventral decubitus. The apical and posterior branches of the PA are usually ligated first, followed by the bronchus and other remaining PA branches. The pulmonary vein is the last structure to be ligated. This approach is more challenging when the hilum access is involved. Nevertheless, anatomical challenges during lung resection can be reduced after the initial experience. Indeed, this single-stage approach is preferred to two-stage surgical approaches when the spine is involved.
Despite the high morbidity rate, this study showed that en bloc resection and spinal stabilization through a single-stage posterior approach for patients with T4 Pancoast tumors invading the spine could be successfully performed without 90-day mortality. In previous studies, the mortality rate of patients with Pancoast tumors and underwent surgery ranged between 0% and 9.6% [14,23,24,26–28].
2. Adjuvant therapy
Only four patients (22.2%) in our series received adjuvant chemotherapy, which was consistent with previous studies showing that most patients with Pancoast tumors are unfit because of the additional toxicity of chemotherapy after a major surgery [28,30]. This study could not reveal if adjuvant chemotherapy was a significant factor influencing the survival of patients with T4 Pancoast tumors invading the spine because of the limited sample size. However, on the basis of previous studies, which revealed the benefit of adjuvant chemotherapy in patients with non-small cell lung cancer after surgery [31,32], we favored prescribing adjuvant therapy to this subset of patients. Further studies with a larger sample size should still be conducted to verify the benefit of adjuvant therapy in patients with Pancoast T4 tumors invading the spine and underwent surgical resection.
3. Survival and tumor control
Seven patients (38.9%) experienced tumor recurrence, which was locoregional in four patients (22.2%) and distant in three patients (16.7%). In previous studies, the recurrence rate ranged from 13.3% to 64.8% [14,22,24,27,28].
The mean survival time of this series was 67 months (95% CI, 43–90 months). The 2-year actual survival of our series was 59% (95% CI, 35.7%–82.3%), which was similar to that of previous series (47%–79%) [14,22,23,26,27]. Our study revealed that the 5-year actual survival rate was 52.5%, which was better than that of the series of patients who did not receive induction chemoradiation (27%–36.2%) [14,23,26] and comparable with that of the series of patients who underwent this treatment (44%–63%) [24,27,28]. Although definitive conclusions cannot be made solely on the basis of these data compared with other published series, these findings support the current recommendation of trimodality therapy over other therapeutic combinations in treating patients with locally advanced Pancoast tumors without mediastinal nodal involvement [24,28,33].
Several limitations were noted in our study. First, this study was retrospective, so it was prone to recall and misclassification biases. Second, to our knowledge, although this study had the largest series of patients who had T4 Pancoast tumors and underwent en bloc resection combined with spinal stabilization through a single-stage posterior approach, the number of included patients was limited (N=18). However, in the context that only few reports about this subject are available, our study provided practical information on the efficacy and safety of this technique. Lastly, the liability of survival analysis might be influenced because 10 patients are still alive at the end of the study.
Conclusions
As the largest series, this study showed that en bloc resection combined with spinal stabilization through a single-stage posterior approach might be effective for treating T4 Pancoast tumors with spinal involvement.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: Zhi Wang, Daniel Shedid, Van Tri Truong; administrative support: Daniel Shedid; provision of study materials or patients: Moishe Liberman, Daniel Shedid, Zhi Wang, Sung-Joo Yuh, Ghassan Boubez; collection and assembly of data: Van Tri Truong, Fidaa Al-Shakfa, Émilie Renaud-Charest, James Wu, Tarek Sunna; data analysis and interpretation: Fidaa Al-Shakfa, Van Tri Truong; and manuscript writing: all authors.