Early Phase Functional Recovery after Spinal Intramedullary Tumor Resection Could Predict Ambulatory Capacity at 1 Year after Surgery
Article information
Abstract
Study Design
This is a single-center retrospective cohort study with a university hospital setting.
Purpose
This study aims to evaluate the short-term course of physical function and walking ability after intramedullary spinal cord tumor (ISCT) resection and predict walking independence 1 year after surgery.
Overview of Literature
Although several reports have shown the postoperative functional prognosis of spinal intramedullary tumors with long-term follow-up, no reports have identified the predictors associated with the functional outcome at an early stage.
Methods
A total of 79 individuals who underwent ISCT resection at our institute between 2014 and 2019 were enrolled in the study, whose preoperative walking state was independent ambulator regardless of cane support with the Functional Independence Measure Locomotor Scale (FIM-L) score of ≥6. The FIM-L, the American Spinal Injury Association (ASIA) motor and sensory scores in the lower extremities, and the Walking Index for Spinal Cord Injury II (WISCI II) were assessed for walking independence, lower-limb function, and walking ability, respectively. These evaluations were performed at 4 time points: preoperatively, 1 week (1W), 2 weeks (2W), and 1 year after surgery.
Results
In the early phase after surgery, 71% and 43% of the participants were nonindependent ambulators at 1W and 2W, respectively. Histopathology indicated that patients with solid tumors (ependymoma, astrocytoma, or lipoma) showed significantly lower indices at 1W and 2W than those with vascular tumors (hemangioblastoma or cavernous hemangioma). Regarding tumor location, thoracic cases exhibited poorer lower-limb function at 1W and 2W and poorer walking ability at 2W than cervical cases. According to the receiver operating characteristic (ROC) analysis, 2 WISCI II points at 2W had the highest sensitivity (100%) and specificity (92.2%) in predicting the level of walking independence at 1 year postoperatively (the area under the ROC curve was 0.99 (95% confidence interval, 0.93–1.00).
Conclusions
The higher the lower-limb function scores in the early phase, the better the improvement in walking ability is predicted 1 year after ISCT resection.
Introduction
Primary spinal cord tumors are among the rarest, accounting for about 2%–4%, tumors originating from the central nervous system [1,2]. Among them, intramedullary spinal cord tumors (ISCTs) have a low incidence of 5%–15% of all spinal cord tumors. The majority (70%–85%) are gliomas, i.e., ependymomas and astrocytomas, with a higher frequency of ependymomas in adults and, conversely, astrocytomas in children [3,4]. Other histological tumors include hemangioblastoma and cavernous hemangioma. There are risks of functional deterioration involved in surgical treatment for ISCT [5]. To mitigate the risk of postoperative neurological deterioration, earlier surgery before developing severe myelopathy is deemed necessary [6,7]. The objective of the surgery is to prevent paralysis from worsening rather than improving it.
Several reports have examined the postoperative functional prognosis of ISCTs [7–10]. However, they are mainly long-term longitudinal studies, and the timing of functional evaluation is not constant. No reports have examined the short-term course of physical function in the early postoperative period. In recent years, the length of stay in acute care hospitals has decreased. The hospitalization period after ISCT surgery is also short (2–3 weeks). Therefore, identifying the predictors associated with the functional outcome at an early stage is necessary. Physical function and walking ability are particularly important focal points for this. This study aimed to examine the short-term course of physical function and walking ability in patients with ISCTs in the early postoperative period, which could predict the ambulatory state 1 year after surgery and would be helpful for planning the postoperative rehabilitation program with a long-term perspective.
Materials and Methods
1. Subjects
This study was approved by the Ethics Committee of the Keio University School of Medicine (approval no., 20110142). We obtained written informed consent for the use of patient data from each patient or their guardian, according to the hospital’s ethics guidelines.
Approximately 432 consecutive patients who underwent spinal cord tumor resection surgery at Keio University Hospital between 2014 and 2019 were retrospectively reviewed. The clinical data for each patient was collected through medical records in accordance with the institution’s ethical guidelines. Among these cases, 161 patients presented with cervical and thoracic ISCTs. All surgeries for ISCT resection were performed via posterior approach microscopically by the same skilled surgical team under general anesthesia with neuromonitoring. Exclusion criteria included preoperative gait disorder (Functional Independence Measure Locomotor Scale (FIM-L) score of 5 or less [11]), a history of brain disease or lower-limb injury, and postoperative complications that delayed the start of rehabilitation. The reason for excluding nonindependent preoperative ambulators was that preoperative physical function had been reported to affect postoperative function [7,8,12,13]. This study focused on the course of physical function and walking ability after surgery and the effects of rehabilitation in independent preoperative ambulators. Consequently, 79 total independent ambulatory preoperative cases (44 males and 35 females) were eligible for a series of follow-up and neurological examination for 1 year postoperatively. The mean age was 46.8±16.2 years (range, 11–81 years) (Table 1).
2. Postoperative physical therapy program
On the first day after surgery, the bed angle was up to 30°. The bed angle was then limited to 60° on the second postoperative day, and the wheelchair transfer began on the fourth postoperative day. To prevent rupture of the sutured dura and arachnoid maters, our standard postoperative course after spinal cord tumor resection is to gradually stand for 4 days instead of leaving the bed immediately. The practice in the rehabilitation room began 1 week after surgery. Physical therapy in the rehabilitation room consisted of range-of-motion exercises, muscle strengthening, basic movement exercises, walking exercises, and endurance training according to each patient. Treatment frequency was 20–40 minutes a day, 5 days a week.
3. Outcome measures
Postoperative functional status at 1 and 2 postoperative weeks was evaluated using the following measurements: the American Spinal Injury Association (ASIA) lower extremity motor score (ASIA-LEMS) [14] was used for lower-limb motor function, the ASIA lower extremity sensory score (ASIA-LESS) [14] below the L1 level for lower-limb sensory function, the Walking Index for Spinal Cord Injury II (WISCI II) [15] for walking ability, and the FIM-L score for walking independence [11]. WISCI II was developed for patients with incomplete spinal cord injuries [16] and evaluated walking function on a 21-point scale based on walking aids, orthotics, and physical assistance. However, the definition of the grading of physical assistance is unclear. This is the part where those who do not need a walking aid but need physical assistance are rated higher than those who need a walking aid but do not need physical assistance. Ditunno et al. [17] stated that WISCI II scores should not be dichotomized into dependent and independent levels of physical assistance. Therefore, to assess for physical assistance for walking, FIM-L was used. The FIM-L score is assigned on a 7-point scale based on walking assistance: 1, full assistance; 2, maximum assistance; 3, moderate assistance; 4, minimum assistance; 5, monitoring; 6, modified independence (with equipment); and 7, independence without equipment. In this study, an independent ambulator was defined as FIM-L score of ≥6 and nonindependent as FIM-L score of <5.
First, the above indices collected 1 and 2 weeks after surgery were compared between independent and nonindependent ambulators, whose walking ability was evaluated 1 year postoperatively. Moreover, the above indices were compared using tumor histology and localization (cervical or thoracic spinal cord). For the grouping of tumor histology, ependymoma, astrocytoma, and lipoma were classified as solid tumor group, and hemangioblastoma and cavernous hemangioma were classified as vascular tumor group. We examined changes in walking ability from 2 weeks to 1 year postoperatively in 78 patients who could be assessed with FIM-L and WISCI II at 1 year postoperatively. Then, the cutoff value for the discrimination of walking independence at 1 year postoperatively was calculated using the receiver operating characteristic (ROC) curve from the WISCI II score at 2 weeks postoperatively.
4. Statistical analysis
The normality of all data was confirmed using the histogram. The clinical course of all subjects was analyzed, and the results were compared according to walking independence, tumor histology, and tumor location. The Wilcoxon signed rank test was performed to compare continuous variables within a subject, while the Mann-Whitney U test was performed to compare them between subjects. Fisher’s exact test was performed for walking independence at 2 weeks postoperatively. A univariate logistic regression was performed to predict walking independence (independent ambulators defined as FIM-L score of ≥6) at 1 year postoperatively from the WISCI II score at 2 weeks after surgery. The optimal cutoff value of the WISCI II score was calculated based on the Youden’s index of the ROC curve. Then, the sensitivity and specificity were calculated using the optimal cutoff value. For statistical analysis, IBM SPSS ver. 25.0 (IBM Corp., Armonk, NY, USA) was used, and the significance level was set at 5%.
Results
1. Preoperative demographic data
The baseline demographics of the 79 subjects who underwent spinal ISCT resection surgery are summarized in Table 1. The study participants included those who walked independently before the surgery. Therefore, all of the study participants had an FIM-L of at least 6 points and a WISCI II of at least 19 points. The median score of ASIA-LEMS was 50 out of 50. The median score on ASIA-LESS was 33 out of 36. Regarding tumor histology, 46 cases had solid tumors (43 gliomas and three lipomas), and 33 had vascular tumors (23 cavernous hemangiomas and 10 hemangioblastomas) (Table 2).
2. Walking independence and physical functions at 1 and 2 weeks after tumor resection
Fig. 1A–C shows the results for all participants at 1–2 weeks postoperatively. The percentage of nonindependent ambulators decreased from 71% to 43% at 1–2 weeks postoperatively. During this observation period, a significant improvement in lower-limb motor function was observed, but no significant change was found in sensory function. Then, the independent and nonindependent ambulator groups 2 weeks after surgery were compared. Table 3 demonstrated that independent ambulators 2 weeks after surgery showed significantly better lower-limb and sensory functions evaluated by ASIA-LEMS and ASIA-LESS scores at 1 week after surgery than those nonindependent ambulators. At 1 week after surgery, 63% of the nonindependent ambulator group could not walk or could walk only with parallel bars (WISCI II score: 0–4). Conversely, all patients in the independent ambulator group started walking with a walker or a cane (WISCI II score of 8 or higher).
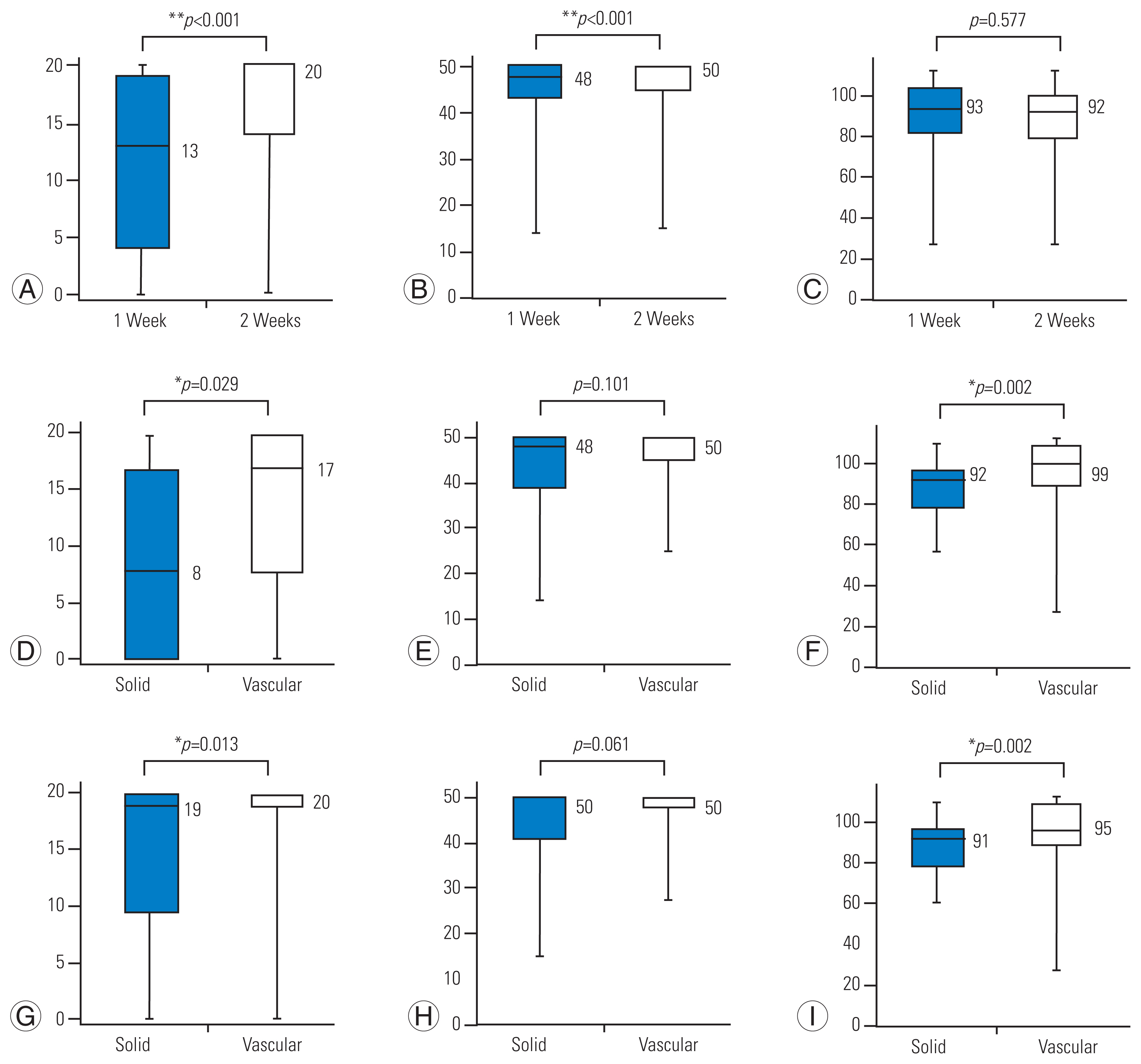
Changes of Walking Index for Spinal Cord Injury II (WISCI II) and American Spinal Injury Association (ASIA)-lower extremity motor score (LEMS) and lower extremity sensory score (LESS). (A–C) Results of all subjects. Comparison of solid and vascular tumor groups at 1 week (D–F) and 2 weeks (G–I) postoperatively. *p<0.05. **p<0.01.
3. Differences in postoperative physical function scores by tumor histopathology
Differences in postoperative walking ability, lower extremity motor function, and sensory function by tumor histopathology are shown in Table 4 (Supplement 1) and Fig. 1D–I. Those with solid tumors exhibited significantly lower walking ability and sensory function than those with vascular tumors, but no statistically significant difference was observed in lower-limb function at 1 and 2 weeks postoperatively (Fig. 1D–I).
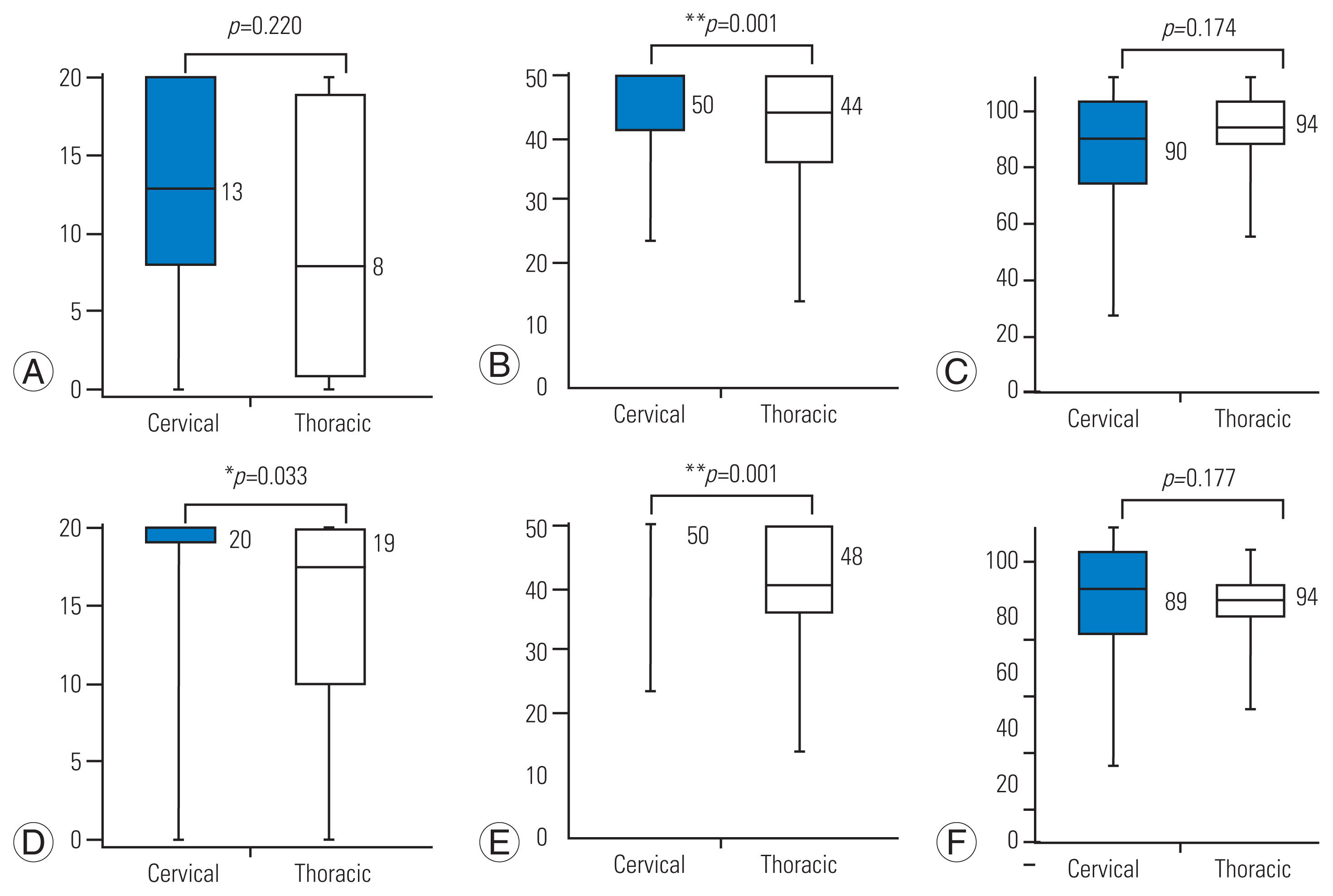
4. Differences in postoperative physical function scores by tumor location
The comparison was conducted focusing on tumor location, cervical or thoracic (Table 5, Supplement 2, Fig. 2). Cases with thoracic tumors were found to have significantly lower-limb function than those with cervical tumors 1 week after surgery. However, no statistically significant differences were found in walking ability and sensory function between the cervical and thoracic locations (Fig. 2A–C). At 2 weeks postoperatively, cases with thoracic tumor showed significantly lower walking ability and lower-limb function, except sensory function (Fig. 2D–F).

Comparison of solid tumor histological types and locations: comparison of cervical and thoracic groups
5. Changes in walking ability from 2 weeks to 1 year after surgery
Finally, the transition of walking ability has been evaluated among 78 individuals who were evaluated by FIM-L and WISCI II, and who were followed 1 year after surgery (Fig. 3). Fig. 3A shows the results at 2 weeks and 1 year follow-up. Nonindependent ambulators decreased from 43% to 10% at 2 weeks and 1 year postoperatively. Fig. 3B shows the change in WISCI II scores from 2 weeks to 1 year postoperatively. At 2 weeks after surgery, 42 patients walked without a cane (WISCI II: 20), 27 patients required assistance or walking aid (WISCI II: 6–19), and nine patients had difficulty walking or walked with parallel bars (WISCI II: 0–2). All 27 patients who needed walking aid or assistance at 2 weeks after surgery recovered as independent ambulators (walking without a cane or without assistance with a cane or brace) 1 year after surgery. Of the nine patients with a WISCI II score of 0–2 at 2 weeks after surgery, one recovered to walk without a cane (WISCI II: 20), one used a cane and no assistance (WISCI II: 19), five used walking aids or assistance (WISCI II: 6–14), one remained with difficulty walking (WISCI II: 0), and one had difficulty walking (WISCI II: 2–0) after reoperation due to recurrence at 1 year after surgery. The cutoff value of the WISICI II score at 2 weeks postoperatively was derived using the ROC analysis to determine the level of walking independence at 1 year postoperatively. The optimal cutoff value of the WISICI II score at 2 weeks postoperatively was 2 points to predict walking independence at 1 year postoperatively according to the Youden index (Table 6, Supplement 3). The area under the curve was 0.99 (95% confidence interval, 0.99–1.00), and the sensitivity and specificity with optimal cutoff value were 100.0% and 97.2%, respectively.
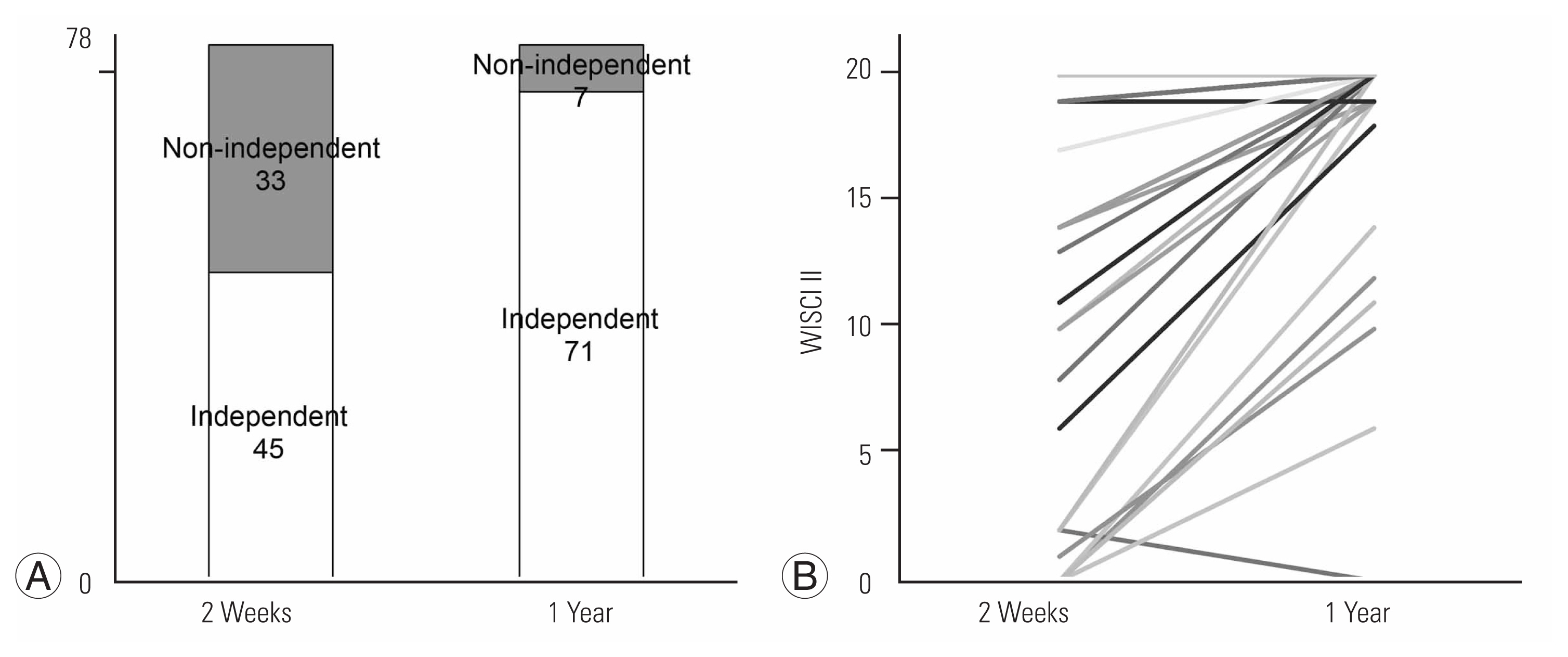
Walking ability at 2 weeks and 1 year postoperatively. (A) Changes in walking independence. (B) Walking Index for Spinal Cord Injury II (WISCI II) from 2 weeks to 1 year.
Discussion
In this study, the postoperative functional status in the early phase (1 and 2 weeks postoperatively) was evaluated using the FIM-L score (walking independence), the WISCI II score (walking ability), ASIA-LEMS (lower-limb function), and ASIA-LESS score (sensory function), and independent walkers at 2 weeks after surgery were found to have better lower-limb and sensory functions recovery at 1 week postoperatively and could walk with a walker or cane. Although there is a risk of motor deterioration and gait disturbance after ISCT resection, preservation of the lower-limb and sensory functions at 1 week postoperatively predicts gradual and steady motor function recovery at 2 weeks postoperatively. In other words, while sensory function was poorly recovered, lower-limb motor function exhibited significantly better recovery, suggesting that improvement in walking ability involved not only lower-limb motor function but also motor coordination. Furthermore, we found that the cutoff value of the WISICI II score at 2 weeks postoperatively was 2 points to predict recovery of walking ability as independent ambulator at 1 year postoperatively (Table 6). Therefore, an earlier start of postoperative rehabilitation for ISCTs is considered necessary to strengthen lower-limb muscles and practice repetitive movements considering sensory disturbance to improve walking ability.
Regarding the difference in surgical outcomes by tumor histopathology, those with solid tumors (ependymoma, astrocytoma, or lipoma) were found to have lower walking ability and sensory function at 1 and 2 weeks after the surgery than those with vascular tumors (hemangioblastoma or cavernoma). A smaller population of people with solid tumors could recover to walk independently at 2 weeks postoperatively. Differences in tumor histopathology could determine the surgical approach, especially for myelotomy procedures. Vascular-type ISCTs, consisting of hemangioblastoma and cavernoma, typically exhibited lateral deviation on axial magnetic resonance imaging images and/or trans-pial exophytic appearance on the microscope and forced the surgeons to perform myelotomy from the dorsal root entry zone or direct trans-pial myelotomy [18,19]. However, ependymomas, accounting for the majority (63%) of the solid tumor group, typically occupy a central location within the spinal cord [20]. In other words, vascular tumors are more likely to be present on the spinal surface, which might contribute to the achievement of less invasive myelotomy maneuvers during tumor dissection and removal; those with vascular tumors showed milder postoperative functional impairment than those with solid tumors. The solid tumor group did not differ from the vascular tumor group in lower-limb motor function but had poor sensory function and inferior walking ability. This may be due to differences in the myelotomy approach.
ISCT surgery is based on the posterior median sulcus approach, which is indicated for most ISCTs, especially ependymomas [21–23]. Postoperative sensory disturbances are common [12]. This sensory disturbance can range from mild to severe, with difficulty standing and walking due to deep sensory disturbance. When deep sensory disturbance occurs, coordination is severely impaired, and walking is often difficult even when lower-limb muscle strength is maintained. The solid tumor group was more likely to develop deep sensory disturbance due to surgical invasion by the posterior median sulcus approach, and improvement in walking ability was poor at 2 weeks postoperatively. Comparing the tumor location, those with tumors in the thorax showed poorer lower-limb function at 1 week postoperatively and lower-limb function and walking ability at 2 weeks postoperatively than those with tumors located cervically. This result was consistent with previous reports of surgical results of intramedullary spinal ependymomas [24,25]. One presumed reason for the fragility of the thoracic cord is the smaller size of the cord than that of the cervical cord [24,26,27], and the other is the poorer blood supply of the thoracic cord [28]. These anatomical factors might have little influence on the tolerability of the thoracic cord to intramedullary surgical procedures.
The optimal cutoff value of the WISICI II score at 2 weeks postoperatively was 2 points to predict walking independence at 1 year postoperatively using ROC analysis (Table 6). WISCI II 2 points are defined as “ambulates in parallel bars, with braces and physical assistance of two persons, 10 meters,” while 3 points indicate “ambulates in parallel bars, with braces and physical assistance of one person, 10 meters.” While previous reports appreciated the importance of preoperative walking ability to achieve a better ambulatory outcome after ISCT resection [5,7,8,10,12,24,25,29], our results suggested that earlier and more aggressive intervention by physical therapy could facilitate long-term recovery of ambulation after ISCT resection.
This study has several limitations. First, evaluating for the sensory function was not enough, especially for position sense and deep sensation. In this study, the ASIA-LEMS score was adopted to quantitatively assess for sensory disturbance. However, it was not suitable for evaluating position sense and deep sensation. Since our results suggested a close relationship between sensory disturbance and postoperative motor impairment, a more detailed evaluation of sensory function is needed. Second, postoperative myelopathic pain should also be considered. Postoperative pain and numbness have been reported to arise often after ISCT resection, and their persistence could impair patients’ quality of life in the postoperative period [30]. Third, the assessment of motor function was exclusively limited to lower-limb functions. This study focused on walking ability and independence; however, not only the lower-limb function but also trunk function plays an important role in walking ability. Therefore, the lack of analysis of trunk functions limited the quality of this study. Although further research is needed, elucidating the characteristics of physical function in patients after ISCT surgery in the early postoperative phase would help predict functional recovery.
Conclusions
By 2 weeks postoperatively, more than 50% of postoperative patients with ISCT surgery regained their level of walking independence. If lower-limb motor and sensory functions are preserved at 1 week postoperatively, an improved walking ability can be expected at 2 weeks postoperatively. The cutoff value of the WISICI II score at 2 weeks postoperatively was 2 points to predict recovery of walking ability as independent ambulator at 1 year postoperatively. Therefore, an early start of postoperative rehabilitation for ISCTs is deemed necessary.
Acknowledgments
The authors thank for Dr. Akio Iwanami for his critical advices. The authors also thank for Ms Makiko Miyazaki, Ms Yukari Yamanishi, and Ms Kie Wakita for their assistance. The final draft of this manuscript was edited by Francois Renault-Mihara (ClearBioEditing).
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
TS, OT, and TF designed the study, and wrote the initial draft of the manuscript. NN, KW, MM, TT, and MN was responsible for designing the study protocol, recruiting participants. RI played an important role in the statistical verification, and contributed to the preparation of the manuscript. MI and MK contributed to analysis and interpretation of data and assisted in the preparation of the manuscript. OT was responsible for all working related to this submission as corresponding author. Also, all authors approved the final version manuscript and agreed to be accountable for all aspects of the work.
Supplementary Materials
Supplementary materials can be available from https://doi.org/10.31616/asj.2022.0068
Supplement 1. Walking independence in solid and vascular tumors.
asj-2022-0068-Supplementary-1.pdfSupplement 2. Walking independence in cervical and thoracic spinal cord tumors.
asj-2022-0068-Supplementary-2.pdfSupplement 3. Receiver operating characteristic curve of Walking Index for Spinal Cord Injury II to determine whether a person can walk independently.
asj-2022-0068-Supplementary-3.pdf