Oblique Lumbar Interbody Fusion with Selective Biportal Endoscopic Posterior Decompression for Multilevel Lumbar Degenerative Diseases
Article information
Abstract
Oblique lumbar interbody fusion is a minimally invasive procedure for treating degenerative lumbar disease. Its advantages include correcting coronal and sagittal spinal alignment and indirect neural decompression. However, achieving a successful outcome is limited in some patients who need direct decompression for central canal lesions including hard stenotic lesions (endplate or facet articular osteophytes and ossification of posterior longitudinal ligaments) and sequestration of the disk. Biportal endoscopic spinal surgery is a minimally invasive technique, which directly decompresses the lesion. By taking advantage of two procedures, in a long-level lumbar lesion, alignment correction and direct decompression can be both achieved. Herein, the authors introduce multilevel lumbar fusion through oblique lumbar interbody fusion and selective direct decompression through biportal endoscopic spinal surgery and discuss the surgical indications, surgical pitfalls, and recommendations for application. Consequently, it is regarded as a minimally invasive interbody fusion method for patients with multilevel lumbar degenerative degeneration.
Introduction
Since the introduction of minimally invasive retroperitoneal anterior lumbar interbody fusion, otherwise called lateral lumbar interbody fusion (LLIF) in 1997, oblique lumbar interbody fusion (OLIF) has become a standard lumbar interbody fusion technique that can overcome the shortcomings of conventional posterior lumbar arthrodesis [1,2]. Compared with direct LLIF through the transpsoas approach, OLIF through the anterior-to-psoas approach does not involve dissecting and splitting of the psoas muscle, which prevents lumbar plexus injury, thereby reducing the risk of postoperative sensory and temporary motor weakness. The oblique lateral corridor in front of the psoas muscle provides safe and easy access to the L2–S1 intervertebral disk with minimal psoas retraction [3]. However, whether indirect neural decompression alone by OLIF is sufficient in some patients with a space-occupying lesion in the lumbar neural foramen remains controversial [4,5].
Meanwhile, biportal endoscopic posterior decompression (BEPD) through direct neural decompression by the minimally invasive posterior interlaminar or paraspinal approach has some advantages such as early postoperative rehabilitation, shorter hospital stay, and less immediate postoperative pain than open decompressive laminectomy [6,7]. Through endoscopic visualization and continuous saline irrigation, fine discrimination under highly magnified views is possible. The indications of BEPD are nearly identical to that of the conventional open posterior approach [8].
In selected patients in whom multilevel lumbar fusion surgery with coronal and/or sagittal deformity correction is required, we believe that combining two minimally invasive procedures, OLIF and BEPD, is the best practice to restore global spinal alignment and obtain direct neural decompression. Thus, we aimed to illustrate some cases to describe the surgical techniques and highlight the advantages of this combined surgery.
Technical Note
1. Patient selection
This retrospective case series study was approved by the Institutional Review Board of Hallym University Kangnam Sacred Heart Hospital (IRB no., 2022-04-014). Written informed consents were obtained from the patients. Five patients with multilevel (more than three levels) lumbar spondylosis with lumbosacral radiculopathy who received OLIF and BEPD between February 2019 and April 2021 were included in this study. All patients received a two-stage surgery. First, OLIF was performed, and magnetic resonance imaging (MRI) was taken 2 days postoperatively. Thereafter, a second-stage surgery was performed 5 days postoperatively. Selective decompressive laminectomy using the biportal endoscopic technique for the insufficient levels of central canal decompression on postoperative MRI and percutaneous pedicle screw fixation were performed. All procedures were performed by a single orthopedic spinal surgeon. The inclusion criteria were as follows: three or more levels of degenerative lumbar disease between the L2 and S1 which was refractory to >3 months of conservative treatment, a space-occupying stenotic lesion in the lumbar spinal canal and/or neural foramen confirmed on MRI, degenerative lumbar scoliosis, and global sagittal imbalance. The exclusion criteria were as follows: other pathological conditions such as acute fracture, infection, or tumor; inaccessible approach for OLIF, such as vascular anatomy or previous abdominal surgery; and requiring spinopelvic fixation in patients with osteoporosis (if there is no percutaneous system).
2. Surgical steps
1) Step 1: anesthesia and patient position
After induction of general anesthesia and appropriate intravenous line access, the patient was placed on a bendable surgical table in a right-sided lateral decubitus position. A bendable table was useful to approach the space between the 12th rib and iliac crest. After confirming the position by fluoroscopic guidance, the patient was draped in a sterile fashion.
2) Step 2: OLIF
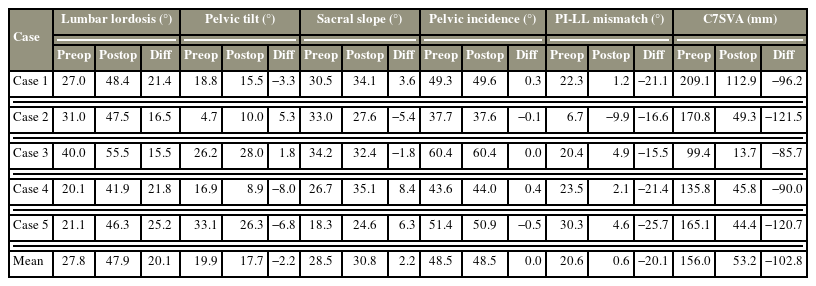
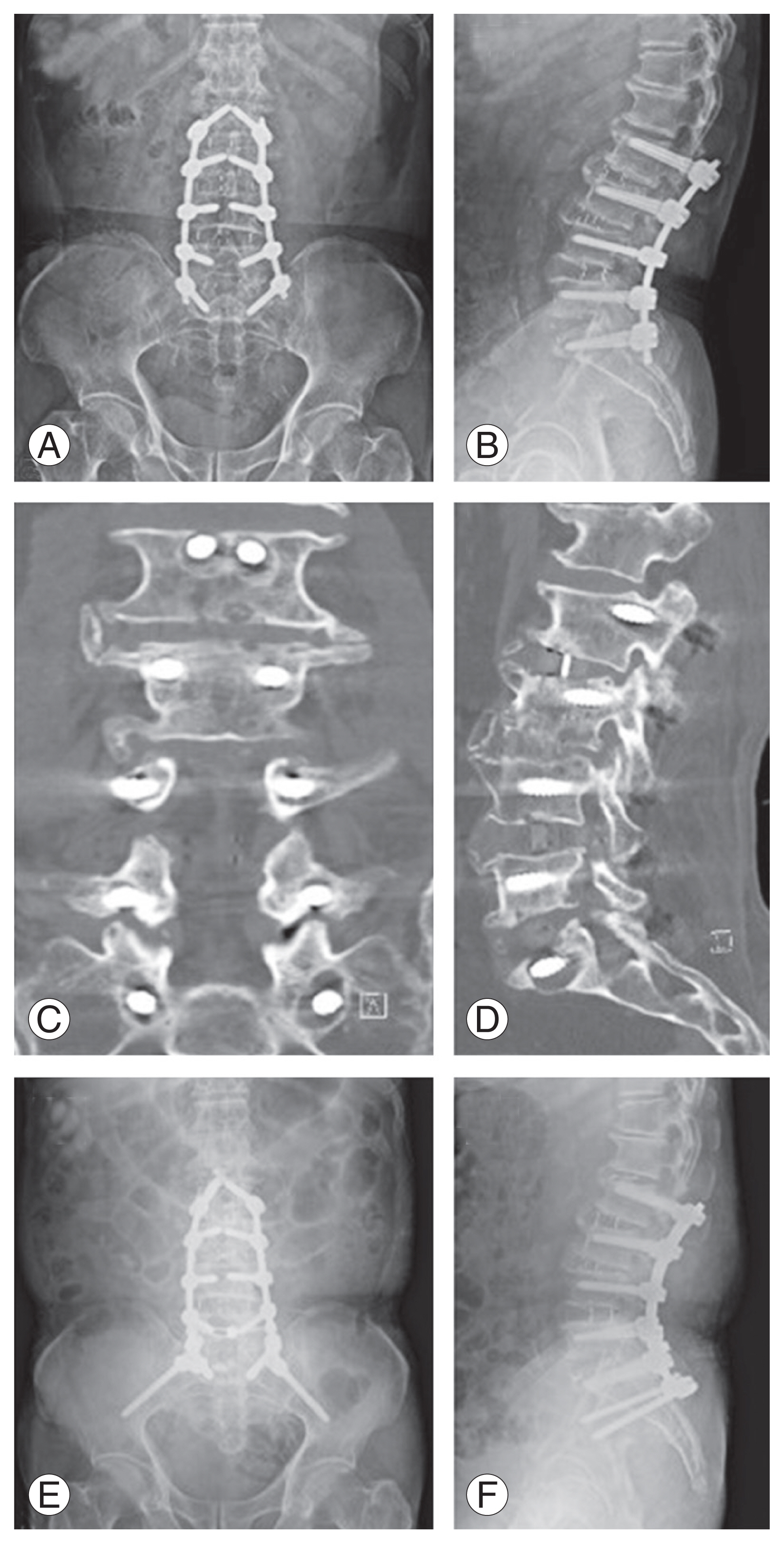
After confirming the target disk level under fluoroscopy, the skin incision sites were marked. For multilevel OLIF from the L2 to S1, three incisions were needed for the L2–3, L3–4–5, and L5–S1. Each curved-linear skin incision of approximately 4–5 cm was made, centered on the target segment and parallel to the external oblique muscle. In the case of L5–S1, the procedure was performed similarly to the previously introduced method (Fig. 1A) [9]. Subsequently, blunt dissection of the external/internal oblique abdominal muscle fascia along the direction of the muscle fibers and transverse abdominal fascia was made. After blunt dissection of the retroperitoneal space, the peritoneum and major vessels were mobilized ventrally. Then, the intervertebral space and surgical corridor between the abdominal aorta or left common iliac vessels and psoas muscle was palpated, and a tubular retractor system was applied (Fig. 1B). First, annulotomy and discectomy were performed, followed by endplate preparation. Contralateral annulotomy was performed using the Cobb elevator. After the endplate preparation, the size of the interbody cage was determined by the size of the trial cage under fluoroscopy (Fig. 1C, D). An appropriately sized Clydesdale PEEK cage (Medtronic, Minneapolis, MN, USA) was filled with demineralized bone matrix (Grafton; Medtronic). Subsequently, cage insertion was performed in the lateral oblique direction, proceeding through the true lateral direction using the rotational orthogonal maneuver. The proper positions of the cages were confirmed under fluoroscopy (Fig. 1E, F). Sufficient saline irrigation and bleeding control were performed, and the abdominal muscles were sutured by layer, completing the operation.
Procedures of the multilevel oblique lumbar interbody fusion. (A) The incision line was determined under fluoroscopy. (B) After dissection, tubular retractor system is settled. (C, D) Suitable cage size is selected by cage trial under fluoroscopy. (E, F) Subsequently, the cage was inserted in an appropriate location under fluoroscopy.
3) Step 3: biportal endoscopic posterior decompression
As a timed scheduled surgery, if the radiating pain and central canal stenotic lesion did not remarkably improve on postoperative MRI, selective-level biportal endoscopic direct posterior decompression was planned. In the second operating procedure, after general anesthesia was induced, the patient was placed prone on the Jackson frame. Then, surgical draping in a water-proof, sterile fashion was completed.
Skin incisions for biportal endoscopic portals were marked surgically under fluoroscopy (Fig. 2A). For BEPD, two 0.7-cm skin incisions were each made approximately 1 cm above and 1 cm below the interlaminar space approximately in the lateral aspect of the spinous process. Generally, for a right-handed surgeon, the left-side incision served as a viewing portal, and the right-side incision was used as a working portal [10]. In multilevel surgery, the incision could be minimized using the viewing portal at the upper level and the working portal at the lower level. The saline infusion pressure was <40 mm Hg during the surgical procedure. After endoscopic visualization of the interlaminar space (Fig. 2B), ipsilateral hemilaminotomy was performed, followed by a ligamentum flavectomy to the contralateral possible extent (Fig. 2C). Adequate neural decompression was confirmed when the ipsilateral and contralateral traversing nerve roots were identified with the free movement, and discectomy was performed if necessary (Fig. 2D).

Procedures of multilevel selective posterior decompression by biportal endoscopic spinal surgery. (A) The incision line was determined under fluoroscopy. In this case, the right-side approach was made. (B) The lamina and interlaminar space were exposed after creating a working space. (C) Ligamentum flavectomy after hemilaminotomy. (D) Subsequently, thecal sac and both traversing roots were well decompressed.
4) Step 4: percutaneous pedicle screws and rod fixation
By using the percutaneous pedicle screw and rod insertion system, pedicle screws were inserted percutaneously under C-arm fluoroscopic guidance and by making additional small skin incisions. After rod fixation, the wound was closed.
3. Results
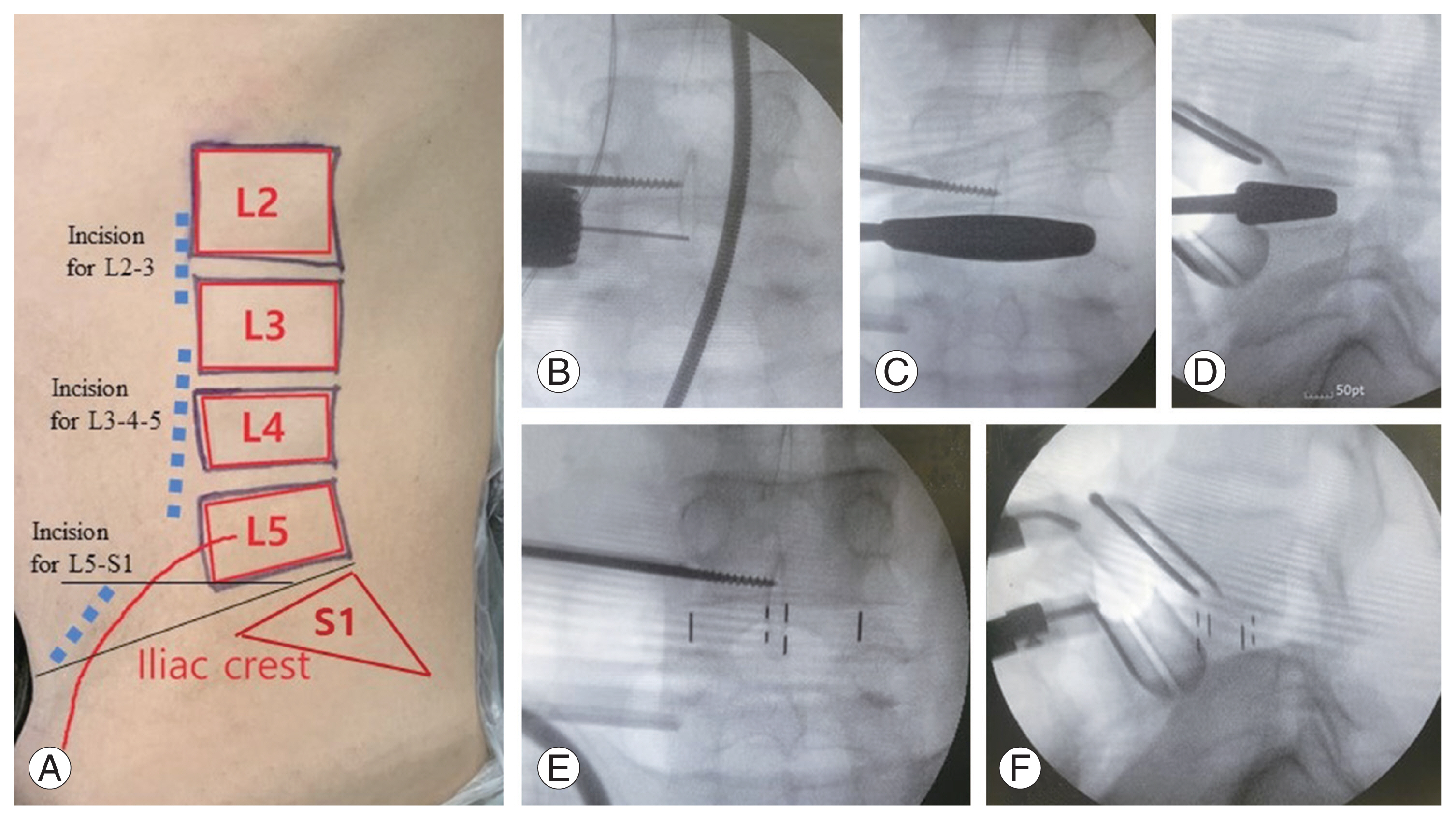
In this study, multilevel OLIF with selective BEPD was conducted in four men and one woman with a mean age of 74 years. Of the cases, three required four levels of surgery, and two required three levels. The average hospital day was 12.8 days; average operative time, 379 minutes; average estimated blood loss (EBL), 470 mL (range, 250–800 mL); and average surgical drainage volume, 352 mL (range, 249–424 mL). Blood transfusion was performed at an average of 1.2 times with 400 mL of packed red blood cells. The average serum hemoglobin decreased by 2.0 (12.4–10.4) (Table 1). On average, lumbar lordosis increased from 27.8° preoperatively to 47.9° postoperatively. Accordingly, the pelvic incidence minus lumbar lordosis mismatch decreased from 20.6° preoperatively to 0.6° postoperatively, and the C7 sagittal vertical axis also decreased from 156.0 mm preoperatively to 53.2 mm postoperatively, resulting in the correction of sagittal alignment (Table 2 and Figs. 3, 4). No major complications were recorded in the perioperative periods, although one case of pseudoarthrosis was noted on the L5–S1. The patient complained of persistent bilateral buttock pain, and pseudoarthrosis on the L5–S1 was observed on computed tomography (CT) at 12 months postoperatively. Consequently, iliac screw fixation was performed, and the pain was relieved (Fig. 5).
Radiographic illustration of one case. Preoperative whole spine radiographs (A, B) indicate positive sagittal imbalance (C7 sagittal vertical axis 209.1 mm) and decreased lumbar lordosis (27.0°). Preoperative axial T2 magnetic resonance image (MRI) revealing the L2–3 (C) and L3–4 (D) with severe central canal stenosis with epidural lipomatosis. After oblique lumbar interbody fusion, postoperative radiographs (E, F) and MRI indicated that the L2–3 (G) and L3–4 (H) remained unaffected by the stenotic lesions. After biportal endoscopic posterior decompression and percutaneous pedicle screw fixation, a postoperative whole spine radiograph (I, J) indicates restoration of sagittal balance and lumbar lordosis (48.4°), and the MRI reveals the decompressed lesion of the L2–3 (K) and L3–4 (L).

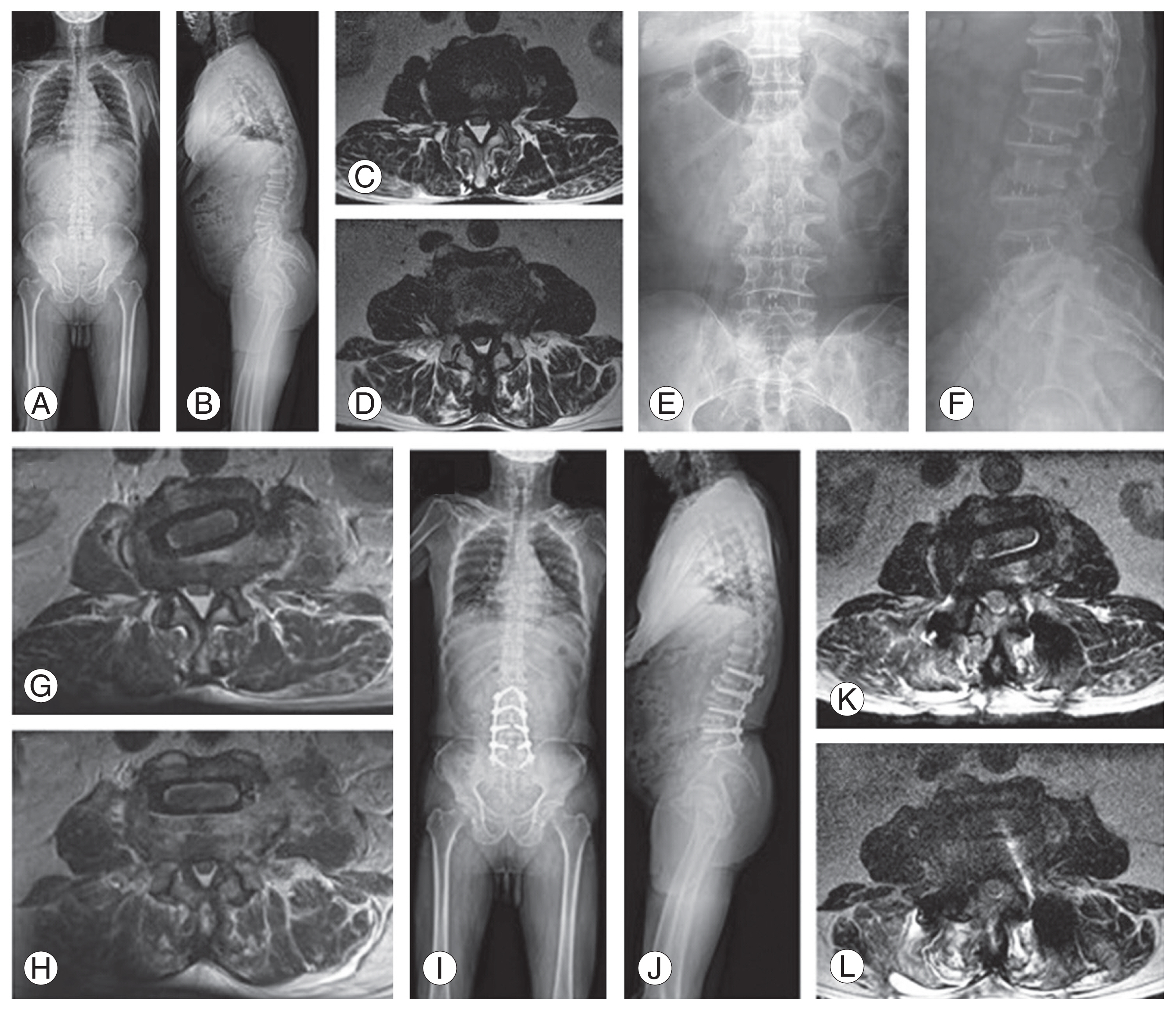
Radiographic illustration of another case. (A, B) Preoperative whole spine radiographs indicate positive sagittal imbalance (C7 sagittal vertical axis 99.4 mm). (C) Preoperative axial T2 magnetic resonance image (MRI) reveals that the L2–3 had severe central canal stenosis and the L4–5 (D) with a right paracentral herniated intervertebral disc. After oblique lumbar interbody fusion, (G) the MRI revealed that the L2–3 was slightly decompressed, although radiculopathic symptoms still existed, and (H) the disc herniation in the L4–5 remains unchanged. After biportal endoscopic posterior decompression and percutaneous pedicle screw fixation, (I, J) the postoperative whole spine radiograph reveals restoration of sagittal balance and increased lumbar lordosis (40.0°→55.5°). The MRI reveals the decompressed lesion of the L2–3 (K) and L4–5 (L).
Discussion
LLIF has comparably favorable long-term surgical outcomes with posterior lumbar interbody fusion (PLIF), although LLIF has better performance in multilevel surgery, such as less blood loss, shorter operative time, and better restoration of global spinal alignment, than PLIF [11,12]. OLIF for degenerated lumbar kyphoscoliosis is less invasive than other lumbar interbody fusion procedures, and good surgical results have been reported without major complications [13]. By using a different approach from the previous posterior surgery, adhesions from the previous surgery will not be encountered, making OLIF a good option for revision surgery [14–17].
However, OLIF has some limitations. In the cases of hard stenotic lesions including lateral recess and foraminal stenosis by osteophyte formation, calcified disk or posterior endplates, ossification of the posterior longitudinal ligament, synovial cysts, and severe central stenotic lesion, indirect decompression via OLIF alone may not be sufficient [4,5,18,19]. Although Ohtori et al. [20] reported that ligamentum flavum buckling is released and remodeled after indirect decompression and interbody fusion, a longer time is required. Hence, whether severe central canal stenosis by thickened ligamentum flavum may be contraindicated to indirect decompression remains controversial. Hayama et al. [21] reported that after they determined the insufficient neural decompression status through intraoperative CT myelogram, they performed further direct posterior decompression and achieved favorable results. Additionally, Li et al. [18] reported that extremely severe lumbar central canal stenosis is the main indication for second-stage posterior direct decompression after LLIF. Similarly, we assessed whether the space-occupying stenotic or disk lesion remains in the spinal canal or neural foramen after indirect decompression by OLIF on postoperative MRI. If the space-occupying lesion remains, and the patient’s symptoms are consistent with the lesion, we planned to direct neural decompression by BEPD. In our cases, hard stenotic lesion and ligamentum flavum thickening could be removed by BEPD, and the patients’ symptoms were relieved. Therefore, BEPD is a good option for direct neural decompression in these cases.
BEPD has some advantages, such as better immediate postoperative clinical outcomes, early functional outcomes, less intraoperative blood loss, and accessibility for any posterior lesion such as central canal stenosis and foraminal stenosis [6,7]. Moreover, endoscopic visualization of the surgical field has some advantages of reducing damage to neurovascular structures and displaying hidden areas that are difficult to be identified with conventional microscopic surgery [22]. Lee et al. [23] have reported that OLIF with BEPD in a single-level lumbar disease demonstrated less blood loss than transforaminal lumbar interbody fusion (TLIF) and improved clinical outcomes postoperatively without a difference in operative time. The mean operative time required for additional endoscopic decompression was reported to be only 50 minutes without major complications. In this study, our operative time was between 50 and 80 minutes at each level, and no major perioperative complications including cardiopulmonary dysfunction occurred.
The main notable aspect of this minimally invasive hybrid technique is the low intraoperative and postoperative blood loss. In the previous results of EBL after lumbar interbody fusion [11,12,24–27], LLIF and OLIF were reported to be in the low 100 mL range, minimally invasive TLIF in the mid 200 mL range, and open TLIF in the mid 500 mL range per operation level. Despite the small number of cases in the series, the average EBL in this study was 470 mL and 130.6 mL per operation level, which is slightly higher than that of LLIF and OLIF. In addition, the average Hemovac drainage volume and number of blood transfusions were lower than those of other lumbar interbody fusions [28,29]. Therefore, this technique may be more useful in older patients with poor conditions and many underlying diseases requiring minimized perioperative blood loss.
This study had some limitations. First, this was a single-institution retrospective study with a small number of cases. Second, the follow-up period was insufficient to present the surgery outcomes. Finally, we investigated patients with moderate coronal and sagittal imbalance and without osteoporosis. Thus, confirming the results through a multicenter prospective study with a larger number of cases is necessary.
In conclusion, OLIF with selective BEPD is a feasible option. Specifically, considering 3–4 levels of lumbar interbody fusion in older people, this minimally invasive hybrid technique is expected to restore segmental coronal and sagittal spinal alignment and allow direct neural decompression to the necessary level while reducing the risk of perioperative complications.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: MSK, HJP; data curation: KHY, HJP; formal analysis: WML, JHK; funding acquisition: not applicable; methodology: HJP; project administration: HJP; visualization: WML; writing–original draft: WML, KHY; writing–review & editing: all authors; and final approval of the manuscript: all authors.