Preemptive Caudal Ropivacaine: An Effective Analgesic during Degenerative Lumbar Spine Surgery
Article information
Abstract
Study Design
This was a prospective, randomized, controlled trial comprising 60 patients undergoing lumbosacral spine (noninstrumentation/nonfusion) surgery.
Purpose
The purpose of this study was to evaluate the efficacy of 0.2% ropivacaine (20 mL) administered alone as a single, preoperative, caudal epidural block injection versus that of intravenous analgesics in providing effective postoperative analgesia to patients undergoing lumbosacral spine surgery.
Overview of Literature
Various studies have shown the effectiveness of a caudal epidural injection (bupivacaine or ropivacaine) in providing postoperative analgesia in combination with steroids or other analgesics. This study uniquely analyzed the efficacy of a single injection of caudal epidural ropivacaine in providing postoperative pain relief.
Methods
Sixty patients who were scheduled to undergo surgery for degenerative lumbar spine disease (noninstrumentation/nonfusion) were consecutively divided into two groups, group R (Study) and group I (Control). 30 group R patients received a caudal epidural block with 20 mL of 0.2% ropivacaine after the administration of general anesthesia. 30 group I patients received no preoperative analgesia. Intravenous analgesics were administered during the postoperative period after a complaint of pain. Various parameters indicating analgesic effect were recorded.
Results
There was a significant delay in the average time to the first demand for rescue analgesia in the study group, suggesting significantly better postoperative pain relief than that in the control group. In comparison with the control group, the study group also showed earlier ambulation with minimal adverse effects. The requirement for intraoperative fentanyl was higher in the control group than that in the study group.
Conclusions
Preemptive analgesia with a single epidural injection of ropivacaine is a safe, simple, and effective approach, providing better postoperative pain relief, facilitating early mobilization, and decreasing the intraoperative requirement for opioid administration.
Introduction
The central nervous system (CNS) is considered plastic or modifiable [1]. Pain transmission through peripheral nerves leads to plasticity of the CNS, resulting in more prolonged and pronounced pain perception even after the cessation of painful stimuli [1]. Preemptive analgesia is administered before the onset of pain to prevent CNS plasticity, thus providing more effective pain relief [123].
High degrees of postoperative pain after spinal surgery preclude early mobilization, leading to a prolonged hospital stay [45]. Thus, the principle of preemptive analgesia can be effectively applied to posterior lumbosacral spine surgery.
The most common method to provide pain relief after lumbosacral spine surgery is the parenteral administration of analgesia. During lumbosacral spine surgery, caudal analgesia can be effectively used, although it is not routinely used. Caudal injection through the sacral hiatus is easy to perform; because the injection site is away from the surgical site, such an injection does not increase the risk of either cerebrospinal fluid (CSF) leakage or infection [678]. A single caudal epidural injection administered at least 20 minutes before the initiation of surgery is a relatively safe, simple, and effective method to provide postoperative pain relief. Local anesthetic agents introduced into the epidural space adhere to the nerve root in about 20 minutes and effectively prevent the perception of a nociceptive stimulus [9], blocking CNS neuroplasticity and allowing satisfactory pain relief for the first 24 hours after surgery [123].
Ropivacaine, a new amino amide, is a long-acting, local anesthetic agent. Previous studies have suggested that ropivacaine produces a sensory blockade similar in duration to that obtained with an equipotent dose of bupivacaine [1011]. A clinically adequate dose of ropivacaine appears to be associated with a lower incidence and grade of motor block, and a faster regression of motor block that stimulates earlier mobilization than bupivacaine. Studies have shown that ropivacaine demonstrates obvious sensory-motor separation, producing sensory nerve Aδ and C fiber blockage, while leaving the motor function of Aα fibers largely unaffected [12]. Ropivacaine has also a better safety profile than other local anesthetics in terms of both CNS and cardiac toxicity [13]. Hence, ropivacaine is an ideal drug for lumbosacral spine surgery, as it will not prevent an assessment of the motor system during the immediate postoperative period, apart from causing a significant decrease in postoperative pain, leading to early mobilization.
The overall benefit of intravenous (IV) analgesia versus caudal analgesia is still controversial, although many studies have shown that caudal epidural analgesia provides superior postoperative analgesia compared to IV analgesia [141516]. Presently, no studies have analyzed the efficiency of preemptive ropivacaine as a stand-alone caudal analgesic agent for use in lumbosacral spine surgery. The aim of this study was to compare the efficacy of caudal ropivacaine analgesia versus no preemptive analgesia in degenerative lumbosacral spine surgery.
We evaluated the efficacy of 0.2% ropivacaine (20 mL), administered alone as a single, preoperative, caudal epidural block injection versus no preemptive analgesia during lumbosacral spine surgery (nonfusion/noninstrumentation) with the following objectives:
(1) Calculate the time to the first demand for rescue analgesia.
(2) Analyze the visual analogue scale (VAS) score for the first 24 hours after surgery.
(3) Calculate the time to first ambulation.
(4) Analyze hemodynamic changes.
(5) Monitor the adverse effects of the drug.
Materials and Methods
1. Source of data collection
The study was conducted at the Vydehi Institute of Medical Science and Research Centre, Bangalore, from December 2014 to June 2015. The study was approved by the Institute's ethics committee.
2. Study design
This was a prospective, randomized, controlled trial comprising 60 patients undergoing lumbosacral spine (noninstrumentation/nonfusion) surgery.
3. Sample size
The study included 60 patients who satisfied the inclusion and exclusion criteria, and were scheduled to undergo lumbosacral spine surgery (noninstrumentation/nonfusion) for degenerative spine disease. (Based on previously published studies on VAS scores for two groups, to detect a minimum difference of 2.0 in the VAS score with 90% power and a 5% level of significance, the sample size should be 58. To optimize our results, we chose 60 patients, with 30 in each group.)
4. Inclusion criteria
– Patients scheduled for lumbosacral spine surgery (degenerative disease not requiring fusion or instrumentation)
– Patients between 18 and 65 years of age
5. Exclusion criteria
– Patients younger than 18 or older than 65 years
– Patients with a history of uncontrolled hypertension, diabetes, or asthma
– Patients on long-term corticosteroids
– Patients with preoperative opioid consumption
– Patients who underwent a previous lumbar spine surgery
– Patients with a contraindication to regional anesthesia
– Patients with a hypersensitivity to ropivacaine
– Patients requiring fusion or instrumentation, or with a nondegenerative spinal pathology (tumor, trauma, or infection)
The 60 selected patients were divided into two groups. The patients were randomized using a computer-generated program into the control and study groups.
Group R (study group): The 30 patients in this group received a 20-mL caudal epidural block of 0.2% ropivacaine after general anesthesia.
Group I (control group): The 30 patients in this group received no preemptive analgesia. They received IV analgesia when they were awake and complaining of pain.
6. Methods
A detailed evaluation, including history of previous medical illnesses, previous surgeries, a general physical examination, and appropriate baseline investigations, was performed.
Informed written consent was obtained from each patient after explaining the procedure. During the preoperative visit, all patients became familiar with how to use the VAS, where 0 is no pain, and 10 is maximum or worst pain (Fig. 1).
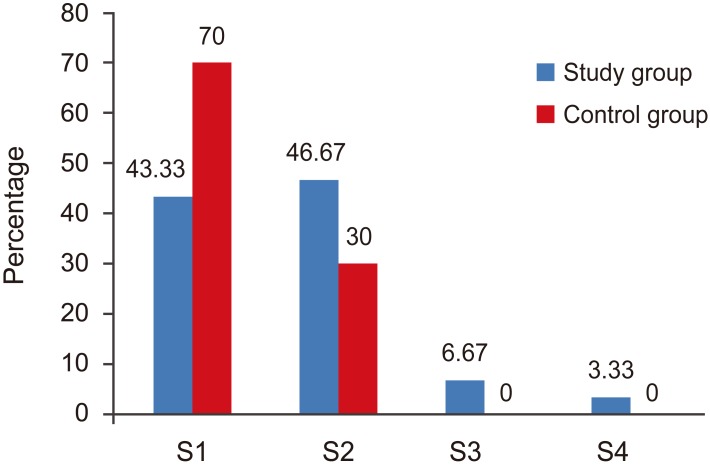
Type of surgery in both groups. S1, laminectomy; S2, femestration and disectomy; S3, hemilaminectomy and disectomy; S4, fenestration, foraminotomy.
All patients fasted 6 hours prior to surgery. After transportation to the operating theatre, standard monitors were attached and baseline vital signs were recorded. An IV line was secured and fluid was infused. General anesthesia (1 µg/kg fentanyl IV bolus) was administered to all patients. Patients were then placed in the prone position on a padded bolster. Patients in the study group (group R) received 20 mL of 0.2% ropivacaine as a caudal block at least 20 min before the initiation of surgery to allow sufficient time for drug action. The control group (group I) did not receive a caudal block; this group also did not receive any saline or other agent to control for the introduction of a comparable epidural volume. This decision was made to avoid possible infection but can be considered a limitation of the study. Intraoperatively, if the pulse rate and/or blood pressure increased, IV fentanyl was administered at an incremental dose of 25 µg.
The duration of anesthesia (the time from when the caudal block was administered to the duration of surgery) was noted. Intraoperatively, patients were monitored for heart rate and blood pressure at regular 10-minute intervals. If blood pressure decreased more than 20% from baseline, then the patient received 500 mL saline infusion; if no response was seen, then 5 mg epinephrine was administered. If heart rate decreased to 45 beats per minute, then an IV injection of 0.6 mg atropine was administered. Postoperatively, VAS scores were recorded by resident doctors who were unaware of which group the patients were in. Hence, this crucial aspect of the study was considered blind.
The following factors were assessed.
(1) Anesthesia time
(2) Intraoperative heart rate and blood pressure
(3) Postoperative pain assessment according to VAS score and hemodynamic parameters at 0, 2, 4, 6, 8, 12, and 24 hours
(4) Time to rescue analgesia (IV analgesia administered after surgery when the VAS score was 3 or higher)
(5) Time taken to ambulation
(6) Incidence of postoperative nausea/vomiting and urinary retention;
IV injections of pentazocine (Fortwin) and/or diclofenac sodium (Dynapar) were administered as rescue analgesic agents.
7. Statistical analysis
Descriptive statistics, such as the mean and standard deviation, were calculated. An unpaired Student's t test was used to determine the statistical significance of differences (significant at p<0.05). A p-value less than 0.05 was considered as indicating a significant difference between the means of the two groups. A p-value less than 0.001 was considered highly significant.
Results
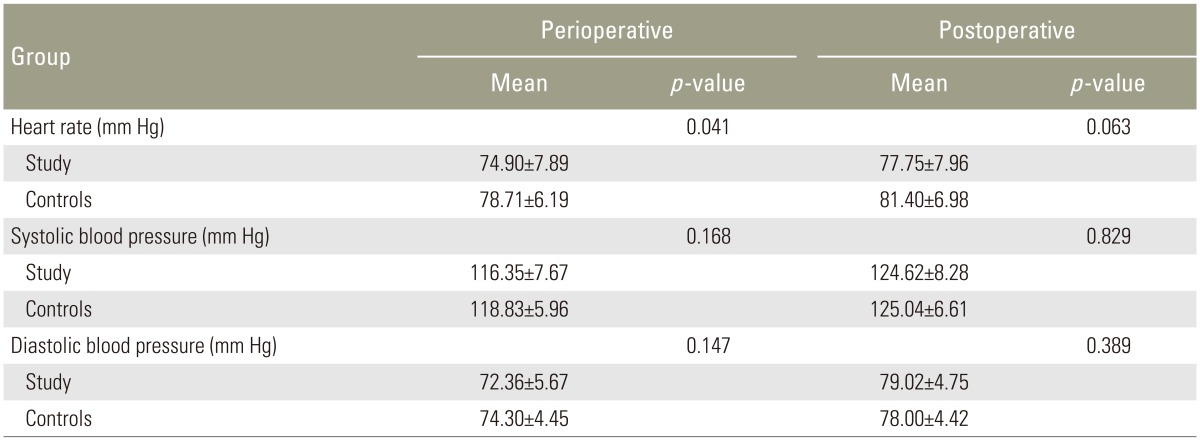
The average ages of the study patients were 44 in group R and 45 in group I. Most patients were between the ages of 30 and 50 years, with 35 male subjects and 25 female subjects. There were no statistically significant differences in patient characteristics with regard to age, gender, height, weight, duration of surgery, or time of anesthesia (Table 1).
The type of surgery was comparable in both groups (Fig. 1).
The total amount of intraoperative fentanyl required was higher (average 171 µg) in the control group than that in the study group (average 143 µg), but this difference was not statistically significant.
There was a statistically significant decrease in heart rate in the study group during the perioperative period compared with the control group. There was also a decrease in blood pressure in both groups (which was greater in the study group) during the perioperative period, but this decrease was not statistically significant (Table 2).
There were no significant changes in hemodynamic parameters in either group during the postoperative period (Table 2).
The VAS scores were statistically lower in the study group during the immediate postoperative period, and at 2, 4, and 6 hours after surgery than in the control group, whereas at 8, 12, and 24 hours after surgery, even though the VAS score was lower in the study group, it was not statistically significant (Fig. 2).
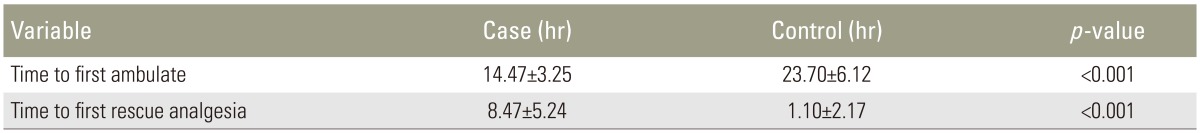
The time to first ambulation was significantly earlier in the study group than in the control group (Table 3).
The time to rescue analgesia was significantly longer in the study group than in the control group (Table 3).
There were a few cases of hypotension, nausea, and vomiting in the study group. However, urinary retention, nausea, and vomiting were more common in the control group (Table 4).
Discussion
Patients who undergo spinal surgery with general anesthesia suffer from considerable wound pain immediately after surgery [1718].
Patients undergoing lumbar surgery, with or without posterior instrumentation, experience severe pain during the postoperative period, which may increase the incidence of postoperative morbidity and complications. Adequate early pain relief hastens rehabilitation and reduces the incidence of chronic pain [19].
Caudal epidural analgesia is an underutilized preemptive analgesia in adults undergoing lumbosacral spine surgery.
Ivani et al. [20] concluded that caudal bupivacaine provides excellent analgesia during the early postoperative period, as is preferable for lumbosacral spinal surgery performed through the posterior approach. In such cases, patients are already placed in the knee-chest position after anesthesia, which is ideal for the palpation and identification of the entry point for anesthesia.
In our study, we administered 20 mL of 0.2% ropivacaine via the caudal epidural route 20 minutes prior to the initiation of lumbosacral spine surgery.
The hemodynamic parameters showed an insignificant decrease in blood pressure and heart rate in the study group both during the perioperative and postoperative periods.
The intraoperative requirement for fentanyl was higher in the control group, which may be due to the direct effect of an increased requirement for intraoperative analgesia. These findings are consistent with those of other studies [2122].
There was a significant delay (p<0.001) in the average time to the first demand for rescue analgesia during the postoperative period in the study group. The average time intervals at which the first demand for rescue analgesia was made were 8.47 hours in the study group and 1.10 hours in the control group. Similar results were obtained in the study conducted by Sekar et al. [8] using 20 mL of 0.375% bupivacaine and 50 mg tramadol. In their study, the mean time interval at which the first demand for analgesia was made were 7.32 hours and 10.6 hours for the control and study groups, respectively. These values also showed a statistically significant difference (p=0.0041).
The patients in the study group had significantly better postoperative pain relief at all time intervals up to 24 hours, as shown in Fig. 2. The VAS score in the study group was significantly lower during the first 6 hours after surgery, indicating significant pain relief in the study group. In the study of Sekar et al. [8], the VAS score was significantly lower in the study group than in the control group. Similar results were obtained in the study of Saoud et al. [23].
Regarding the time of ambulation, patients in the study group showed significantly earlier ambulation then the control group. This may be due to better analgesia in the study group from the caudal analgesia, allowing the patient to get out of the bed easier. Similar results were obtained in the study of Saoud et al. [23].
In our study, side effects such as nausea, vomiting, and urinary retention were observed more frequently in the control group than in the study group. This may be due to the amount of pain and postoperative narcotic consumption. There were a few cases of intraoperative hypotension and bradycardia in the study group, which may be due to the effects of caudal analgesia, but these findings were not significant. Additionally, sympathetic vasoconstrictor fibers run from the segmental levels of T1 to L2, and neural blockade of L2 or higher produces a sympathetic block that manifests as hypotension. Hypotension after caudal analgesia indicates that the segmental distribution is to the upper lumbar or lower thoracic level. A block of T1 to T4 fibers manifests as bradycardia. The distribution of the anesthetic drug within the epidural space is related to a variety of factors, not all of which can be controlled by the anesthesiologist.
Similarly, in the study of A. Saoud et al. [23], there were no complications specific to the procedure, except for the development of transient postoperative urinary retention.
Conclusions
Compared to patients not receiving caudal analgesia, a single preemptive epidural injection of ropivacaine is a safe, simple, and effective approach to provide postoperative pain relief up to 24 hours, facilitate early mobilization, and decrease intraoperative requirements for opioids, This approach is also ideal for postoperative analgesia in patients undergoing lumbosacral spine surgery, as it is unlikely to cause any significant motor block.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.