Minimally Invasive Microscope-Assisted Stand-Alone Transarticular Screw Fixation without Gallie Supplementation in the Management of Mobile Atlantoaxial Instability
Article information
Abstract
Study Design
Retrospective study.
Purpose
To evaluate the clinico-radiological efficacy of stand-alone minimally invasive transarticular screw (MIS-TAS) fixation without supplemental Gallie fixation in the management of mobile C1–C2 instability.
Overview of Literature
Data evaluating the efficacy and feasibility of MIS-TAS in the literature is scanty.
Methods
Patients with mobile atlantoaxial instability and >2 years follow-up were included and managed by stand-alone TAS fixation using the Magerl technique and morselized allograft without additional fixation. Patient demographics and intra-operative parameters were noted. Clinical parameters (Visual Analog Scale [VAS] and Oswestry Disability Index [ODI]), neurology (modified Japanese Orthopaedic Association [mJOA]), and radiological factors (anterior atlanto-dens interval and space available for cord) were evaluated pre and postoperatively. Computed tomography (CT) was performed in patients who did not show interspinous fusion on X-ray at 1 year, to verify intra-articular fusion. Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA); the Student t-test and analysis of variance were used to assess statistical significance (p <0.05).
Results
A total of 82 consecutive cases (three males, one female; mean age, 36.26±5.78 years) were evaluated. In total, 163 TASs were placed. Significant improvement was noticed in clinical (mean preoperative VAS=7.2±2.19, postoperative VAS=3.3±1.12; mean preoperative ODI=78.3±4.83, postoperative ODI=34.05±3.26) and neurological features (mean preoperative mJOA=14.73±2.68, postoperative mJOA=17.5±2.21). Radiological evidence of fusion was noted in 97.5% cases at final follow-up. Seventeen patients were found to have no interspinous fusions upon X-rays, but CT revealed facet fusion in all patients except in two. Inadvertent vertebral artery injury was noted in three cases.
Conclusions
Stand-alone TAS fixation with morselized allograft provides excellent radiological and clinical outcomes. The addition of a supplementary tension band and structural graft are not essential. This provides the opportunity to avoid the complications associated with graft harvesting and wiring.
Introduction
The atlantoaxial articulation is the segment with maximum mobility in the spine. The high mobility, however, come at the cost of increased chances of instability. Various techniques to stabilize this joint have been well described in literature. Recently, posterior techniques have gained prominence [1-5]. Posterior wiring techniques as described by Gallie [4] and Brooks and Jenkins [2] require external immobilization and are fraught with high complication rates. Modern day instrumentation such as C1–C2 transarticular screw (TAS) fixation and screw-rod construct (SRC) have revolutionized the management of atlantoaxial instability (AAI). The TAS technique was introduced by Magerl and Seemann [5] in 1979 as a supplement to Gallie’s fixation and resulted in fusion rates of up to 98% among patients.
Biomechanical studies evaluating the efficacy of posterior techniques in resisting pure movements reaffirm the superiority of TAS [6]. Sim et al. [7] reported that the C1–C2 TAS construct provided better biomechanical stabilization than the C1 lateral mass (LM)–C2 pedicle screw (PS) construct in flexion/extension only, whereas Guo et al. [8] reported better stabilization in axial rotation only. Li et al. [9], Hott et al. [10], and Ma et al. [11] found no significant differences in the stabilization achieved with the C1–C2 TAS and C1 LM–C2 PS constructs in any degree of motion. Du et al. [12] performed a systematic review and meta-analysis to determine the biomechanical stability achieved by several screw constructs for C1–C2 fixation. They reported that C1–C2 TAS provided better lateral bending than C1 LM–C2 PS and C1 LM–C2 translaminar.
Thus, the literature reveals that stand-alone TAS provides excellent rotational and lateral bending stability but is not as effective at resisting flexion and extension loads. In combination with wiring, all pure movements are effectively resisted. Interestingly, good fusion rates have been reported even when the posterior wiring construct failed [13,14]. However, this method carries the risk of potential injury to the neural or vascular structures owing to the anatomical position and anatomic variation among patients [15]. Thus, during screw insertion, neurovascular safety should be carefully evaluated. We hypothesized that the added stability provided by a structural graft and wiring may not influence fusion rates in the clinical setting. If this is correct, an entire set of complications associated with wires and graft harvesting can be avoided. Instead of autograft, we used abundant morselized allograft from a bone bank for fusion, making this one of the very few studies reporting the outcomes of allograft in stand-alone microscope-assisted TAS fixation. We referred to our technique as minimally invasive TAS (MIS-TAS) because of the principles used to minimize surgical trauma by using smaller exposure and avoiding damage to the C2–C3 interspinous ligament and C2–C3 capsule, by using allograft instead of autograft, and by avoiding use of wires. The objective of this study was to evaluate the clinical and radiological outcomes of a stand-alone MIS-TAS fixation with morselized allograft in the management of mobile C1–C2 instability of varied etiology.
Materials and Methods
1. Study design
Retrospective evaluation of prospectively collected data was performed between 2009 and 2014 after the Institutional Review Board of Bombay Hospital & Medical Research Centre approval (IRB approval no., PRO150900009). All the subjects were selected after taking informed consent from a single facility. All the subjects were from a single facility and were operated on by a single surgeon. Two independent observers evaluated the medical records, specifically hospital charts, operating room notes, and the radiological data of all the patients undergoing MIS-TAS fixation.
Inclusion criteria were as follows: (1) mobile and reducible C1–C2 instability with no basilar invagination and (2) minimum 24 months follow-up. Exclusion criteria were as follows: (1) irreducible C1–C2 instability; (2) basilar invagination; (3) C1 LM or C2 articular mass destruction; and (4) cervicothoracic kyphosis.
2. Patient evaluation
Demographic data, including the etiology of AAI, age, sex, and cause of symptoms (mechanical and neurological), were collected. Clinical data, including a Visual Analog Scale (VAS) score of 1–10 for pain, the Oswestry Disability Index (ODI) score of 1–100 for disability indices, and the modified Japanese Orthopaedic Association (mJOA) score for neurologic insult, were assessed. Data were collected pre- and postoperatively at 3 months, 6 months, 12 months, and 24 months. The final outcome was graded both subjectively and objectively, using the scoring system given by Odom’s criteria.
Radiological evaluation included the pre- and postoperative assessment of the anterior atlanto-dens interval (ADI), space available for cord (SAC), and screw placement in relation to the anterior tubercle of atlas and fusion on anteroposterior and lateral radiographs. Preoperative Computed tomography (CT) was performed for every patient to determine the vertebral artery (VA) groove in the parasagittal and coronal cuts. A high-riding VA was not a contraindication but a more dorsal approach and a superior trajectory were adopted. Fusion, both intra-articular and interspinous, was assessed at each follow-up via X-ray imaging. If interspinous fusion was not observed at 1 year, CT was performed to assess intra-articular fusion. Intraoperative data recorded was the duration of surgery, blood loss, VA injury, neurological injury, or any adverse event.
3. Surgical technique
The patient was placed in the prone position and the cranium was held in a Mayfield three-point pin fixation device with traction (Fig. 1). The cervical spine was positioned for fracture reduction while simultaneously ensuring that a trajectory for TAS placement was attainable. This required a “military tuck” position. Lateral fluoroscopic imaging was used to confirm the desired alignment prior to connecting the Mayfield head holder system rigidly to the table. A skin incision centering on C2 was made. Only the inferior arch of C1 and C2 was subperiosteally exposed. The C2–C3 interspinous ligament and facet capsule were preserved. The C2 pars interarticularis was exposed and had to be clearly defined. Prior to the initiation of drilling, the C1–C2 joint was opened using a McDonald or Penfield and the joint surface was curetted. The entry point and trajectory were determined on the basis of a retrograde projection of the dorsal most and medial aspects of the C1–C2 joint line on the inferior part of the C2 lamina. A stab incision was made 1 cm lateral to the midline in the T1–T2 region and a drill guide was used. Once the drill guide was in place, a high-speed burr was used to create a starter hole in C2 at the desired entry site for drilling of the TAS. It was essential to drill as dorsally and medially as possible within the pars interarticularis in order to minimize potential injury to the VA. In a high-riding VA, a steeper trajectory was adopted. After drilling to the anterior cortex of C1, the hole was tapped and the screw was passed. The procedure was repeated on the contralateral side.
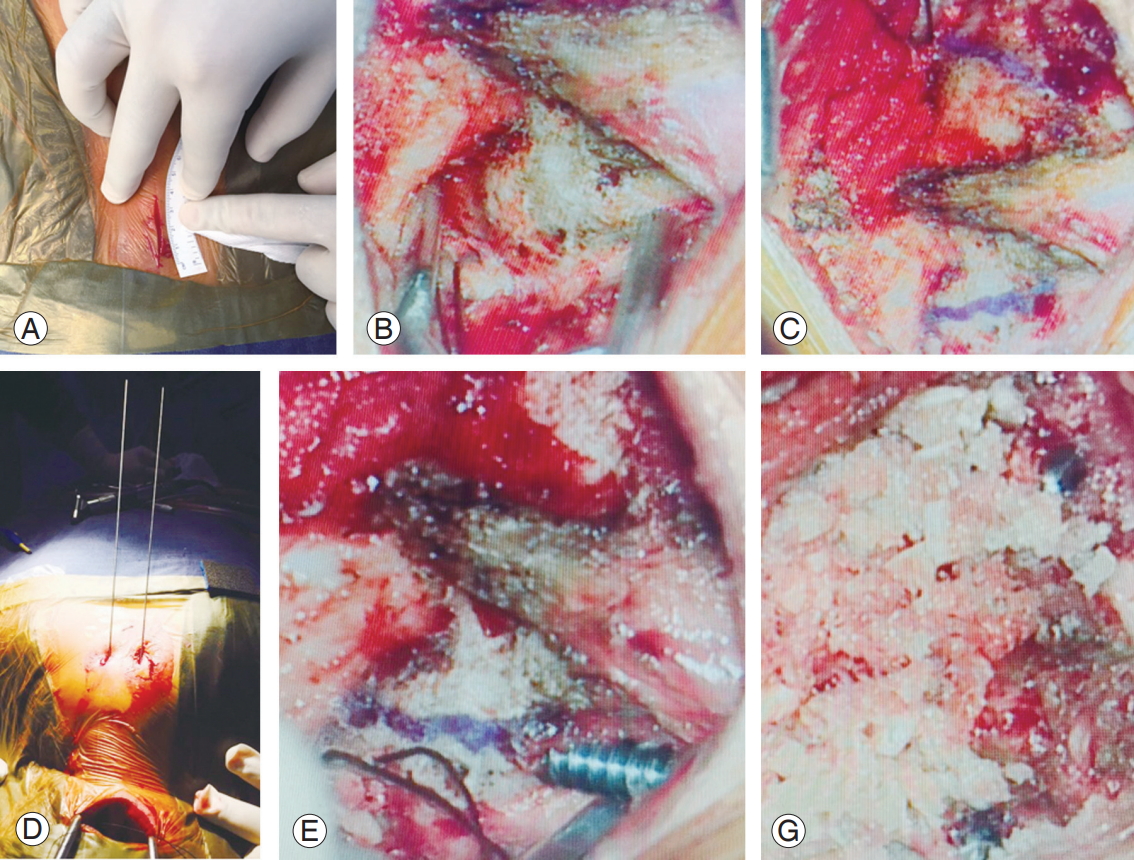
Surgical technique of minimally invasive transarticular screw fixation. (A) Incision of about 3 cm. (B) Exposure of whole extent of lamina and pars. (C) Lines drawn projecting most dorsal and medial part of pars in retrograde manner over inferior part of C2 lamina. (D) Drill guide insertion in percutaneous manner at T1–T2 level. (E) Insertion of screw. (F) Allograft placement between C2 lamina and C1 posterior arch with both the screws inserted.
The lamina of the C2 vertebra and C1 arch were decorticated using a high-speed burr before the application of the bone graft. Freeze-dried allograft from a bone bank was morselized and placed between the posterior arch of C1 and the spinous process of C2 vertebra and along the bilateral facet joints.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA); Student t-test and analysis of variance were used to evaluate any statistical difference as to the postoperative improvement/deterioration of pain and neurological status. Statistical significance was set at p<0.05.
Results
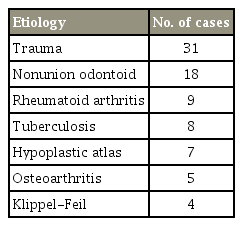
Ninety-three consecutive patients were managed by stand-alone MIS-TAS between 2009 and 2014; six patients with follow-up of less than 24 months and another five patients who were lost to follow-up were excluded from the study. A total of 82 patients with different etiologies formed our study group (Table 1).
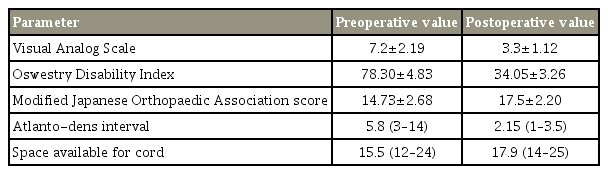
The mean age at the time of surgery was 36.26±5.78 years (range, 20–78 years), and three males and one female; mean follow-up was 38 months (range, 24–60 months). We inserted 163 screws in 82 patients, of which, 10 were cases of high-riding VA. Statistically significant improvement was seen in the mean preoperative clinical parameters at the first follow-up; this was maintained at the final follow-up (Table 2). VAS (7.2±2.19; range, 5–9), ODI (78.3±4.83; range, 64–84), and mJOA (14.73±2.68; range, 11–17) scores improved to 3.3±1.12, 34.05±3.26, and 17.5±2.2, respectively.
Intra-operative blood loss averaged 104.84±21.75 mL (range, 80–350 mL). Operative time averaged 120.11±15.82 minutes (range, 90–210 minutes). In this series, there were no neurological complications but inadvertent VA injury was noted in three cases. In two cases, VA injury occurred while drilling the second screw path, resulting in excessive blood loss that was managed by a tamponade and screw insertion with uneventful sequelae. In one case, it occurred at the time of screw insertion. We managed it with a tamponade using gel foam, abandoned that side screw insertion, and added a wire construct. Postoperative CT angiography of the neck vessels did not show any arteriovenous malformation or aneurysm of the VA with patent flow. No patients showed neurologic deterioration after surgery. One patient experienced a dorsal burst out of the screw in the C2 pars, but the screw was retained with no complications. The mean duration of hospitalization was 7 days (range, 5–11 days).
Both ADI (pre-/postoperation 5.8/2.15) and SAC (pre-/postoperation 15.5/17.9) improved significantly from their preoperative values. All the screws were targeted to the mid-to-upper third of anterior tubercle of atlas. The earliest radiological evidence of union was observed in these patients at a mean follow-up of 4.5 months. X-ray imaging revealed posterior interspinous fusion in 65 patients (Fig. 2). In the remaining 17 patients, the graft was completely absorbed. These patients were evaluated with CT at 1 year which showed intra-articular fusion anteriorly in the facet area in 15 of them, with no movement on dynamic X-ray. The two patients with no intra-articular and posterior fusion mass have completed 48 months follow-up. There has been no implant breakage/loosening and no movement on dynamic films. A 97.5% fusion rate was achieved in this study. Figs. 3 and 4 show intra-articular fusion in postoperative CT at the end of 1 year in two different patients. There was no screw fracture, loosening, or back out at 24 months follow-up. No misplacement of screws has been reported in this study. No re-operation has been reported in any patient at a mean follow-up of 2 years.

36-Year-old male with rheumatoid arthritis with atlantoaxial instability secondary to odontoid nonunion. (A) Preoperative flexion radiograph. (B) Preoperative extension radiograph. (C) Preoperative T2 weighted sagittal image. (D) 3 Months postoperative radiograph showing posterior union. Reduction achieved in hyperextension only.
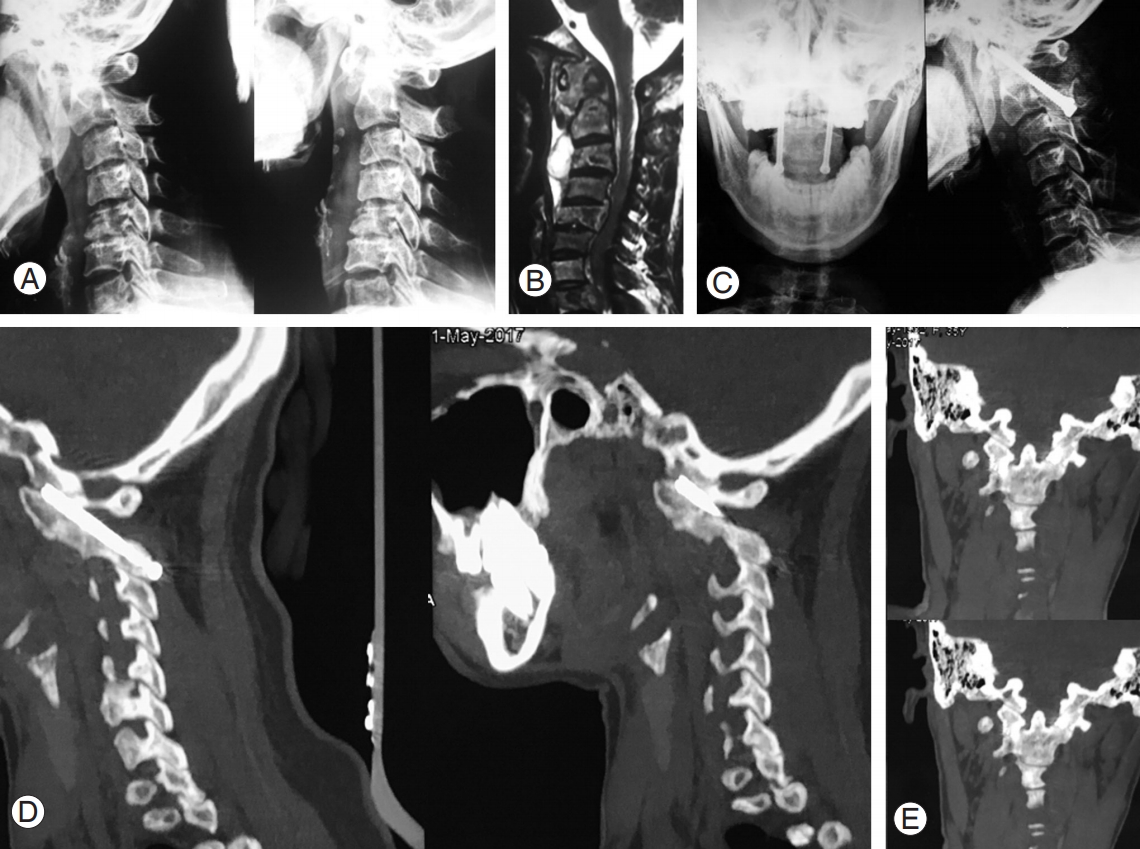
34-Year-old female with atlantoaxial instability secondary to tuberculosis. (A) Preoperative flexion extension radiograph showing atlantoaxial instability. (B) Preoperative magnetic resonance imaging showing destruction of axis with increase soft tissue mass around C1–C2. (C) Postoperative radiograph showing fusion but not extending completely from C1 to C2. (D) Sagittal view of follow-up CT scan showing C1–C2 union. (E) Coronal view of postoperative CT scan showing intra-articular fusion. CT, computed tomography.

20-Year-old male with atlantoaxial instability secondary to nonunion odontoid. (A) Preoperative flexion extension radiograph showing atlantoaxial instability. (B) Preoperative magnetic resonance imaging showing odontoid nonunion and resulting anterior compression over spinal cord. (C) Preoperative sagittal view of CT scan showing nonunion odontoid. (D) Postoperative radiograph showing fusion but not extending completely from C1 to C2. (E) Coronal view of preoperative CT scan showing Odontoid nonunion. (F) Sagittal view of follow-up CT scan showing C1–C2 union. (G) Coronal view of postoperative CT scan showing intra-articular fusion. CT, computed tomography.
In this study, there were no serious complications associated with surgery, including deep infection, neurological deterioration, significant bleeding, or thrombotic events. Moreover, there have been no major clinical problems such as neurologic deterioration because of C1–C2 instability, intractable pain, or screw breakage during the follow-up period.
As per Odom’s criteria, in the current study, 95% of the patients had excellent-to-good outcomes, with complete resolution of symptoms, and three fair and no poor results were noted.
Discussion
TAS fixation with wiring has grown in popularity because of the high fusion rates resulting from a 3-point fixation. We hypothesized that the posterior wiring construct may not be essential for fusion. We arrived at this hypothesis because of multiple reports of sustained excellent outcomes despite wire failure. Matsumoto et al. [14] reported 18 cases (34.6%) of loosening of posterior wiring construct in 52 cases. Despite this, the fusion rate was 95%. Ito et al. [13] reported 100% fusion rates even though all his cases had some degree of loosening, and he concluded that adding a wire construct was not necessary. While the apparent benefits of the posterior wiring construct remain doubtful, the risks associated with its exclusion are quite real. It does not provide sufficient rotational and extension stability; thus, it results in limited fusion rates without external orthosis. Wire breakage and intracanal graft dislodgement have been reported to worsen neurology. Furthering this hypothesis was the study by Wang et al. [16] where 100% fusion rates were achieved in all subjects without any supplementary fixation.
The author used morselized allograft for fusion. While autograft is the ideal graft material with osteoconductive, osteoinductive, and osteogenic properties, harvesting it involves several complications. This is quite extensively covered in the literature and complications ranging from minor ones including superficial infections, and minor hematoma to major ones including herniation of abdominal contents through massive bone graft donor sites, vascular injuries, deep infections at the donor site, neurologic injuries, deep hematoma formation requiring surgical intervention, and iliac wing fractures, and chronic donor-site pain even at 1 year follow-up, have been reported [17-20]. Rates of donor-site morbidity associated with anterior or posterior iliac crest autograft harvesting range from 0.6% to 35% in the literature [18,20]. Robertson and Wray [21] found that in harvesting autograft, major morbidity is due to donor site pain, especially at six months when it is most severe. Silber et al. [22] reported numerous complications, including ambulation difficulty, extended antibiotic usage, persistent drainage, wound dehiscence, re-operation with incision and drainage, cosmetic dissatisfaction, and pain at the donor site. In the present study, we preferred to use morselized allograft from the bone bank to further reduce the complications associated with graft harvesting.
Hillard et al. [23] compared the clinical outcomes between C1–C2 arthrodesis using autograft and allograft. The authors used an interposition strut graft in both the groups and performed fixation with either Magerl’s, Harm’s, or Goel’s technique. They found similar major complication rates (16.7%) associated with the harvesting of posterior iliac crest bone graft for atlantoaxial arthrodesis with greater blood loss and operative time when an autograft was harvested. The fusion rate in their study was >88% and was not statistically different in both the groups. Elliott et al. [24] performed a literature review to compare the results of C1–C2 arthrodesis with autograft and allograft, in which he noted only seven studies using allograft for fusion; the fusion rate was not statistically significant.
A 97.5% fusion rate was achieved in the current study which is comparable to those achieved by other techniques described in literature. Kim et al. [25] compared the clinical and radiological outcomes in patients treated with either TAS technique or by a SRC for C1–C2 arthrodesis. They found a 90% fusion rate for TAS compared to 100% fusion rate for a SRC, but there was no statistically significant difference in functional outcomes between both the groups. Elliott et al. [26] compared the clinical and radiographic outcomes of patients treated with TASs and SRCs for posterior atlantoaxial fusion. The authors found that there was a higher fusion rate in SRCs (97.5%) compared to TAS (94.6%), with a higher incidence of VA in TAS (4.1% versus 2%).
We attribute the high fusion rate in the current study to several factors. In every case, we exposed the C1–C2 joints and curetted the articular surface. In addition to the passage of the guide wire, drilling and passing the screw across the joint causes significant disruption of the joint surface and results in hematoma formation and the release of osteogenic factors. This, coupled with bone-on-bone contact under axial load, favors fusion. The mean duration of radiologically visible fusion using X-rays was 4.5 months. A subgroup analysis revealed a lesser time for fusion in AAI caused by inflammatory and infective conditions as compared to AAI because of trauma. We attributed this to pre-surgical joint erosion resulting from infection and inflammation. The literature suggests that the presence of rheumatoid arthritis entails the risk of posterior graft nonunion more than other disorders [27]. We achieved union in all our nine patients with RA, although six of them had graft resorption posteriorly with anteriorly demonstrable union. This finding is attributable to the careful bone carpentry and facet preparation technique. In patients with rheumatoid arthritis with poor bone quality, the risk of complications associated with graft harvesting may be higher and thus the use of an allograft could be even more beneficial in these patients. None of the other etiologies showed such a pattern of graft resorption.
Surgical time, blood loss, and hospitalization time in the present study were better than those in studies by Ito et al. [13] and Wang et al. [16]. This is likely because, first of all, the time required to add wiring to the construct is saved. Second, use of the allograft alleviated the time and blood loss associated with graft harvesting. Third, the donor-site morbidity resulting in prolonged hospitalization is curtailed. Finally, a smaller incision was used without exposure of the C2–C3 joints or the upper half of C1 the posterior arch.
The incidence of vertebral artery injury (VAI) in TAS has been reported to be 4%–8% [28-30]. Wright and Lauryssen [30] estimated the risk of VAI during C1–C2 TAS fixation to be 2.2% per inserted screw. The risk of neurological deficit from VAI was 0.2% per subject, and the mortality rate was 0.1%. VAI occurred in three cases (3.6%) in this series and highlights the technical challenges involved in TAS surgery. Bleeding associated with VAI was brought under control through tamponade and screw insertion in two cases, whereas only one screw insertion and wiring were necessary in the third case. Though there were no clinical sequelae to the VAI, clinical complications with VAI can be disastrous and have been discussed in detail by other authors.
There was no instrument failure in our cases using 3.5-mm fully threaded titanium screw. We believe the reason for this is that first, all our cases had anatomic reduction without any additional maneuver for reduction resulting in better approximation of joint surfaces. Second, the fusion time was shortened by the use of abundant allograft and thorough recipient site preparation, which is usually not a routine step when one uses autograft due to fear of sublaminar wiring fracturing the decorticated bone. This also shortened the period for which the screw was under stress and minimized the possibility of screw failure.
Thus, careful preoperative planning, good patient positioning to achieve adequate reduction, entry point consideration on the basis of retrograde projection of pars, and facet fusion are factors that must be considered to achieve good results with TAS.
The current study is not without limitations. This was a retrospective study and thus the chance of bias does exist. In addition, we did not have any controls, i.e., other techniques for C1–C2 arthrodesis that could be used to compare the results.
Conclusions
In present series, 97.5% fusion rate was achieved with no surgery-related complications. Thus, stand-alone minimal invasive TAS with morselized allograft have a high fusion rate in atlantoaxial arthrodesis without instrument failure. Although its learning curve may be steep, it is associated with few rates of complications in expert hands.
Notes
No potential conflict of interest relevant to this article was reported.