Repair of Iliac Crest Defects with a Hydroxyapatite/Collagen Composite
Article information
Abstract
Study Design
Retrospective study.
Purpose
This study aimed to assess the effect of refilling with hydroxyapatite/collagen (HAp/Col) composite on an iliac crest defect after spinal fusion.
Overview of Literature
The use of iliac crest bone graft has been the gold standard in spinal fusion for a long time because of its biological and non-immunologic properties. Few reports have addressed how bone defects recover after iliac crest bone harvest following spinal fusion.
Methods
Cancellous bone was collected from the anterior iliac crest during lateral interbody fusion (LIF), and the bone void of the ilium was refilled with a porous HAp/Col composite. We assessed bone recovery using computed tomography (CT). From the 74 patients who underwent LIF between January 2015 and December 2016, we included 49 patients whose iliac crest could be evaluated using CT at 3 months and 1 year after the surgery.
Results
Bone defects decreased in a time-dependent manner after the surgery. Cortical closure was observed in 28.5% of the cases 3 months after the surgery; at 1 year postoperatively, 95.9% of the patients had cortical closure. Complete repair of the cancellous bone was achieved in 57.1% of the patients at 3 months after the surgery and in 95.9% at 1 year after the surgery. There were no significant hematomas, infections, iliac crest fractures, or soft tissue herniation.
Conclusions
Radiographic recovery of cortical and cancellous bone defects was achieved with high probability via refilling with HAp/Col composite over the 1-year period.
Introduction
Lateral interbody fusion (LIF) in lumbar surgery has become an increasingly prevalent option during the previous several years for the treatment of a variety of conditions, including spinal stenosis, spondylolisthesis, and degenerative disc disease [1]. The cages for LIF are larger than those for posterolateral or transforaminal interbody fusion; therefore, this procedure requires a large amount of bone graft between the affected vertebrae to facilitate new bone growth and subsequent fusion.
The use of iliac crest bone graft has long been the gold standard in orthopedic surgery in general, as well as in spinal fusion, because of its superior biological and nonimmunologic properties compared with those of other materials [2,3]. The iliac crest is easy to access and can provide ample bone. Another recognized advantage is the low cost and close to zero risk of disease transmission. However, there are disadvantages in that the bone needs to be harvested from the iliac crest; the surgical time is higher; and there is a risk of infection, hematoma, fracture, and pain [2,4]. Limited bone supply is another problem. Usually, ample bone is available in one posterior crest for one procedure; however, commonly, patients require multiple procedures that need iliac bone harvesting, thus exhausting the supply.
Few studies have reported on how bone defects are treated after iliac crest bone harvest performed at the time of spinal fusion. Burton et al. [5] reconstructed the iliac crest using a hydroxyapatite–calcium triphosphate biphasic compound that improved the body’s ability to reform new bone; however, it did not alleviate the pain. Other studies have investigated the repair of the iliac crest defect using autogenous bone [6], bovine cancellous graft [7], polymethyl methacrylate bone cement [8], allografts fixed with cannulated screws [9], and polymethyl methacrylate bone cement with screws [10]. These methods are problematic in terms of the non-immunologic properties and/or recovery of donor site defects.
A porous hydroxyapatite/collagen (HAp/Col) composite (ReFit; HOYA Technosurgical, Tokyo, Japan), composed of nano-scale HAp and porcine type 1 collagen, is a recently developed material for bone substitute [11,12]. The sponge-like elasticity of HAp/Col is an important characteristic of this graft material because it fits in the space of the bone matrix without any gap, allowing bone formation to extend continuously from the matrix bone [11,13]. Good bio-resorbability, high osteoconductivity, and bone regeneration ability were shown when used to treat defects in the long bones in animal models and in clinical trials [11,12,14,15]. However, the efficacy of the repair of iliac bone defect with HAp/Col composite was unknown.
In this study, we assessed the effect of refilling with HAp/Col composite on iliac crest defects using computed tomography (CT) analysis.
Materials and Methods
1. Subjects
Between January 2015 and December 2016, 74 patients underwent LIF in Kyoto University Hospital, and we included 49 patients whose iliac crest could be evaluated using CT 3 months and 1 year after the surgery.
2. Surgical procedure
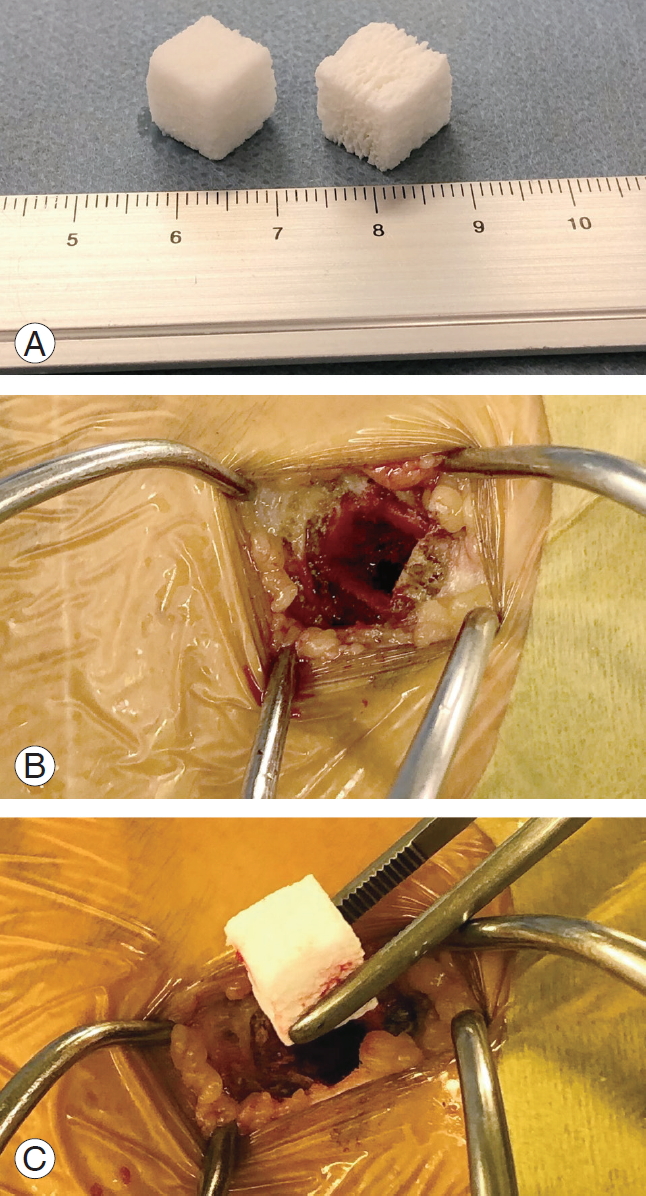
Before approaching the vertebral bodies, bone harvesting was performed. A skin incision was made just above the iliac crest, 3 cm behind the anterior superior iliac spine, and the iliac crest was exposed while preserving the periosteum. A 7-mm square fenestration was performed with a chisel, and the cancellous bone was collected as much as possible with a bone curette. After thorough washing with normal saline, the bone defect was filled with 1- or 21-cm square refits, and then the periosteum, fascia, and subcutaneous tissues were sutured without returning the cortical bone (Fig. 1).
3. Computed tomography assessments
We obtained 0.4-mm-thick axial slices using a 64-line multi-slice CT scanner at 2 weeks, 3 months, and 12 months after the surgery. The maximal diameter of the bone defect was measured using CT images 2 weeks after the surgery. Bone healing was assessed with the images taken 3 and 12 months after the surgery.
4. Statistical analyses
All the statistical analyses were performed with JMP Pro 13 (SAS Institute Inc., Cary, NC, USA) using the two-tailed, paired t-test (two conditions), or one-way repeated measures analysis of variance (multiple comparison) with Tukey’s honest significant difference test as a post-hoc test. Fisher’s exact test was used to compare the proportions.
5. Ethical approval
This study was approved by the Ethics Committee of Kyoto University Graduate School and Faculty of Medicine (E2067). The board waived the requirement for patients’ informed consent because of the retrospective design.
Results
The characteristics of our study subjects are shown in Table 1. The mean age was 68.0 years. There were 38 cases of one-level spinal fusion, 11 cases of two-level spinal fusion, and one case of six-level spinal fusion with the LIF technique (Table 1).
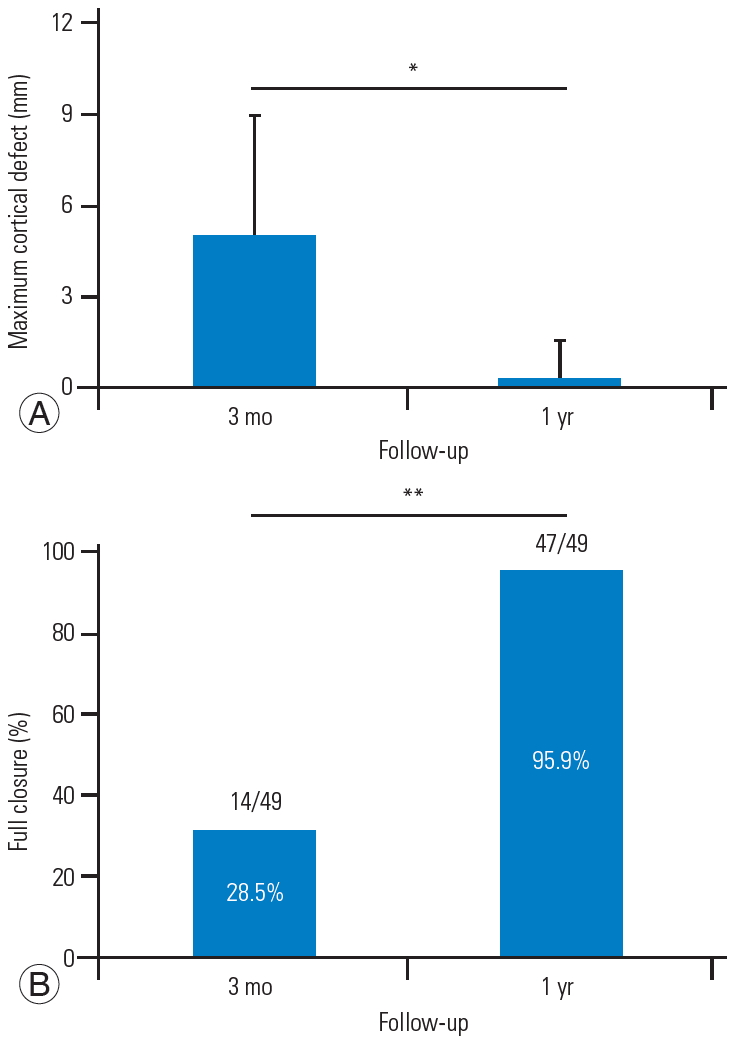
The average maximum diameter of the cortical defects was 5.0 mm at 3 months after surgery and 0.3 mm 1 year after the surgery. In addition, 28.5% of the patients achieved full cortical closure 3 months after surgery, and after 1 year, 95.9% of the patients had cortical closure (Fig. 2).
With regard to cancellous bone defect, an average of 14.5 mm of bone defect was found 2 weeks after the operation. However, the bone defect decreased, and the average bone defect duration was 4.7 mm 3 months after the operation and 0.3 mm 1 year after the operation. Complete repair of the cancellous bone was obtained in 57.1% subjects at 3 months after surgery and 95.9% 1 year after surgery (Fig. 3). Representative cases of bone healing are shown in Fig. 4.
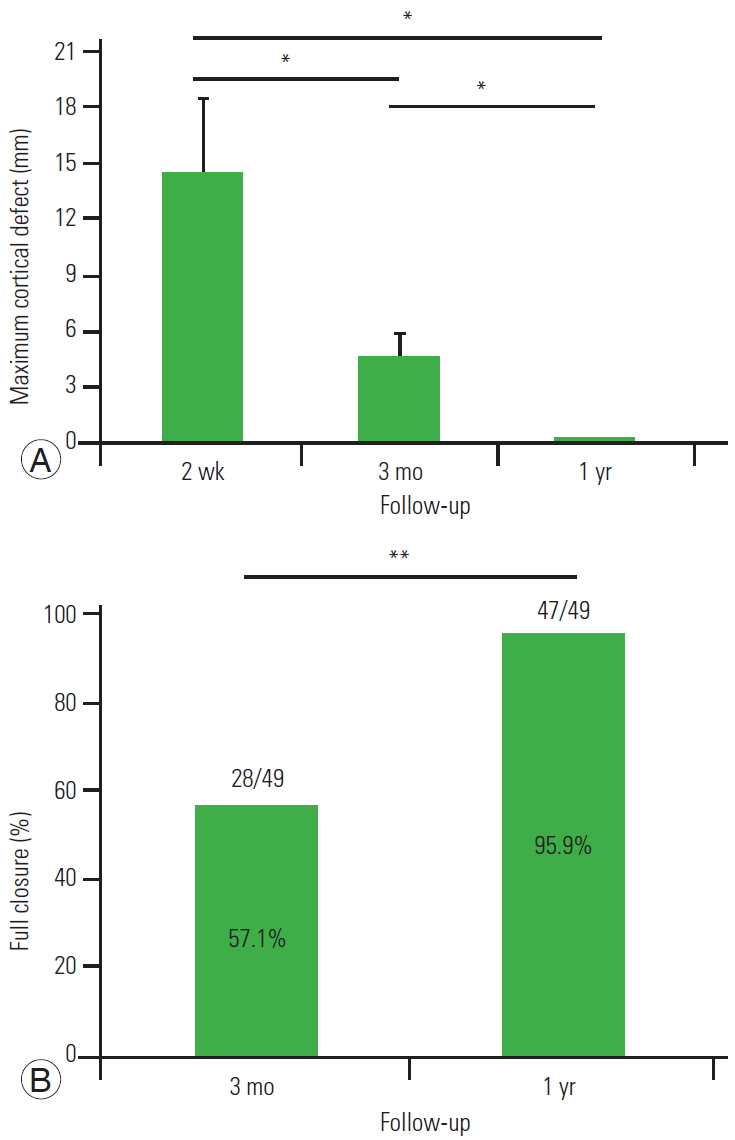
(A, B) Computed tomography analysis of the cancellous defect at the 1-year follow up. *p<0.05. **p<0.01.

Representative computed tomography images in patients with refilling of hydroxyapatite/collagen composite at postoperative 3 months and 1 year. (A, B) 67-year-old woman; (C, D) 52-year-old man.
There were no complications with respect to the bone graft harvest procedure that required re-operation in any patient during the study period. In particular, there were no significant hematomas, infections requiring surgical irrigation or debridement, iliac crest fractures, or soft tissue herniation.
Discussion
In this study, we found a high rate of cortical and cancellous iliac defect repair with HAp/Col composite. Iliac crest autograft has been the gold standard in spinal fusion surgery for a long time because of its osteogenic, osteoinductive, osteoconductive, and non-immunogenic properties [3]. In order to enhance spinal fusion and avoid the morbidity of autograft harvesting, many bone graft substitutes have been developed [2,3,16]. Currently, there are four main classes of bone graft substitute: ceramic-based synthetic grafts, allografts, recombinant human bone morphogenetic proteins, and mesenchymal stem cells. However, large cages are used for LIF, and iliac crest autograft will continue to be used in the near future, though it may be mixed with osteoconductive compounds to increase graft volume and/or reagents to add osteogenic or osteoinductive properties to the graft material [2].
The disadvantage of bone harvest from the iliac crest is that the material may only be harvested once per side. The most common complication associated with iliac crest autograft harvest is pain at the site of the bone collection. There is also a risk of fracture if the cortical bone is harvested.
Ceramic-based synthetic grafts and absorbable collagen sponges are candidates for iliac bone defect refilling [3,16]. Ceramic-based synthetic substitutes, such as hydroxyapatite, tricalcium phosphate, and calcium sulfate, are chemically similar to the inorganic phase of bone and have osteo-friendly characteristics. They also have the advantage of a low risk of disease transmission, easy sterilization, and storage. They are tailored to increase bone in-growth properties, such as pore size and distribution, and scaffold shape and size [17-20]; however, optimal degradation rates have not yet been achieved.
HAp/Col is composed of nano-scale hydroxyapatite (80 weight per weight [w/w] %) and porcine skin-derived atelocollagen (20 w/w %), and its nanostructure resembles that of natural bone [21]. The porous body of HAp/Col, once wet, becomes elastic like a sponge and is thus easy to implant into bone defects of various shapes. This enhances bone conduction continuously from the host bone into the implant by filling the bone defect without gaps. Animal studies have shown vigorous bone formation at sites implanted with porous HAp/Col together with bioresorbability of the implants [22,23].
Currently, limited data are available about iliac defect repair. Burton et al. [5] reported that 43% of their patients had a cortical defect and 84% had a cancellous defect after a 2-year follow-up, when the iliac defect was not filled with bone substitute. With βTCP (MASTERGRAFT), 78% of the patients had full closure of the cortical defect and 61% achieved complete cancellous repair. In this study, a high rate of cortical and cancellous iliac defect repair with HAp/Col composite was attained; 95.9% of the patients had cortical closure, and 95.9% achieved complete cancellous repair, although the high rate of bone repair made it impossible to perform a statistical analysis of the risk factors of incomplete bone repair.
It is noteworthy that there were no significant hematomas, infections requiring surgical irrigation, debridement, iliac crest fractures, or soft tissue herniation. Bone harvest from the iliac crest involves some morbidities. According to a report, superficial hematoma was observed in 1.2%–3.9% of the patients [4]. Deep infection of the iliac donor site is reported to occur in 1.7%–2.5% of the patients [4]. Refilling the donor site defect with HAp/Col composite decreased the dead space and may have reduced the risk of infection. It is also possible that good repair of the iliac donor site with HAp/Col composite reduced the fracture risk.
There are certain limitations to this study: first, this was a single-arm study, and it is unclear what proportion of cortical and cancellous bone defects will occur if the iliac bone donor site is not filled with bone fillers with our way of bone harvest. However, the successful treatment of bone defects using HAp/Col composite was observed in as many as 96% of the patients, and Burton et al. [5] reported that cancellous bone defects were found in 39% of patients without bone fillers, suggesting that refilling with HAp/Col composite was effective. Another limitation is that since the histology has not been examined, it is unclear whether the defect has been completely replaced by the bone and disappeared or if it is ready to be used as a biologically active native bone. However, in the rabbit bone defect model, absorption of the composite and recovery of the bone marrow cavity were confirmed [23].
Conclusions
Treatment of cortical and cancellous bone defects was radiographically completed with a high probability via refilling with HAp/Col composite over the 1-year period. No hematoma or infections were observed. This procedure will be useful when future surgery requires additional bone stock.
Notes
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceived and designed the study: KM, SF; analyzed the data: KM; collection of data and read the radiograph: KM, SF, BO, TS, SM; wrote the paper: KM; supervision of the manuscript: SF, SM; and all authors read and approved the final manuscript.