Assessing the Performance of Prognostic Scores in Patients with Spinal Metastases from Lung Cancer Undergoing Non-surgical Treatment
Article information
Abstract
Study Design
Retrospective study.
Purpose
The purpose of this study was to see how well the Tomita score, revised Tokuhashi score, modified Bauer score, Van der Linden score, classic Skeletal Oncology Research Group (SORG) algorithm, SORG nomogram, and New England Spinal Metastasis Score (NESMS) predicted 3-month, 6-month, and 1-year survival of non-surgical lung cancer spinal metastases.
Overview of Literature
There has been no study assessing the performance of prognostic scores for non-surgical lung cancer spinal metastases.
Methods
Data analysis was carried out to identify the variables that had a significant impact on survival. For all patients with spinal metastasis from lung cancer who received non-surgical treatment, the Tomita score, revised Tokuhashi score, modified Bauer score, Van der Linden score, classic SORG algorithm, SORG nomogram, and NESMS were calculated. The performance of the scoring systems was assessed by using receiver operating characteristic (ROC) curves at 3 months, 6 months, and 12 months. The predictive accuracy of the scoring systems was quantified using the area under the ROC curve (AUC).
Results
A total of 127 patients are included in the present study. The median survival of the population study was 5.3 months (95% confidence interval [CI], 3.7–9.6 months). Low hemoglobin was associated with shorter survival (hazard ratio [HR], 1.49; 95% CI, 1.00–2.23; p=0.049), while targeted therapy after spinal metastasis was associated with longer survival (HR, 0.34; 95% CI, 0.21–0.51; p<0.001). In the multivariate analysis, targeted therapy was independently associated with longer survival (HR, 0.3; 95% CI, 0.17–0.5; p<0.001). The AUC of the time-dependent ROC curves for the above prognostic scores revealed all of them performed poorly (AUC <0.7).
Conclusions
The seven scoring systems investigated are ineffective at predicting survival in patients with spinal metastasis from lung cancer who are treated non-surgically.
Introduction
Lung cancer is one of the most common types of cancer, accounting for 18% of cancer-related deaths in 2020 [1]. The spine is the most frequent site of metastatic lesions in patients with lung cancer [2]. Survival with lung cancer-derived metastatic spine tumors has improved as medical therapies for lung cancer have advanced [3].
A treatment decision for spinal metastases requires the involvement of surgeons, and medical, and radiation oncologists. Treatment must be tailored to each patient’s spinal stability, neurological compromise caused by spinal cord or nerve root compression, tumor histology, performance status, pain, the patient’s systemic condition, and overall prognosis [4]. To predict survival and guide treatment decisions for spinal metastases, several scoring systems that take into account these factors have been developed. The Tomita score [5], original and revised Tokuhashi score [6,7], and original and modified Bauer score [8,9] have been frequently used by spine surgeons to predict survival. The van der Linden prediction model was developed based on a randomized trial of radiotherapy and employs three significant predictors of survival, including primary tumor location, visceral metastases, and functional status [10]. Among these prognostic tools, the revised Tokuhashi and modified Bauer scoring systems were found to have the best predictive accuracy for survival [11]. Ghori et al. [12] demonstrated in 2015 that taking preoperative albumin, ambulatory status, and modified Bauer score into account, this composite model named New England Spinal Metastasis Score (NESMS) was better than the modified Bauer score alone in predicting the 365-day survival with spinal metastases. Skeletal Oncology Research Group recently developed the classic (SORG) algorithm [13] and the SORG nomogram [14] for predicting survival in patients with metastatic spine disease. The NESMS and SORG prognostic tools were reported to be accurate in predicting the 3- and 12-month survival for operable spine metastatic disease [14–16].
Lung cancer with spinal metastases has a poor prognosis. There has been no research into the effectiveness of prognostic scores for non-surgical lung cancer spinal metastases. Before selecting the best candidates for radiation and/or systematic therapy, it is critical to estimate survival. The objective of the present study is to investigate the accuracy of seven prognostic scores, including (1) Tomita score, (2) revised Tokuhashi score, (3) modified Bauer score, (4) Van der Linden score, (5) classic SORG algorithm, (6) the SORG nomogram, and (7) NESMS in predicting 3-month, 6-month, and 1-year survival in this nonoperative population. We also aim to identify the risk factors influencing survival.
Materials and Methods
1. Study population and collected data
From January 2008 to December 2020, we reviewed a database of patients with spinal metastasis from lung cancer who received non-surgical treatment at Centre Hospitalier de l’Université de Montréal. Patients who did not have surgical indications and who had surgical indications but could not undergo surgery because of poor general condition were all included. This research was approved by the ethics committee of Centre Hospitalier de l’Université de Montréal Research Center (approval no., 19.178). Informed consent was obtained from all individual participant included in the study. Medical history, radiographic and/or endoscopic examinations, and pathological results were used to make the diagnosis of lung cancer with secondary spinal metastasis. Patients were followed until death or the latest follow-up by September 30, 2021. For statistical analysis, we divided the following variables into different categories: age (<65 years and ≥65 years), gender (male and female), tobacco use (never smoker and ever smoker), walking ability (intact, impaired, and non-ambulatory), Frankel grade (grade A–D versus grade E), histology of lung cancer (adenocarcinoma, squamous cell carcinoma, small cell, and other), Karnofsky performance scale (KPS) (10%–40% versus 50%–70% versus 80%–100%), and Eastern Cooperative Oncology Group performance (0–2 versus 3–4). The number of spine levels involved with metastasis (single versus multiple), skeletal metastasis (single versus multiple), visceral metastasis, brain metastasis, history of systemic treatment, and radiotherapy of all patients were recorded. Visceral metastases were defined by the following locations: the heart, the lung, the brain, and the organs of the digestive, excretory, reproductive, and circulatory systems. Within 30 days of the diagnosis of spinal metastasis, laboratory results were collected, including hemoglobin levels (<12 g/dL and ≥12 g/dL), platelet counts (<400,000/μL and ≥400,000/μL), and blood albumin levels (<3.5 g/dL and ≥3.5 g/dL). Survival was defined from the date of the spinal metastasis diagnosis to death or the end of the study period (September 30, 2021).
2. Predictive scoring systems
A study member calculated scoring systems such as the Tomita score, revised Tokuhashi score, modified Bauer score, Van der Linden score, classic SORG algorithm, SORG nomogram, and NESMS for all of the patients. The performance of the scoring systems was evaluated using receiver operating characteristic (ROC) curves at 3 months, 6 months, and 12 months. The area under the ROC curve (AUC) was calculated to quantify the predictive accuracy of the scoring systems. If the AUC was less than 0.70, between 0.7 and 0.8, between 0.8 and 0.9, or greater than 0.9, the prognostic score was considered poor, fair, good, or excellent, respectively.
3. Statistical analysis
IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA), SAS ver. 9.1 (SAS Institute Inc., Cary, NC, USA), and R ver. 4.1.2 software (https://www.r-project.org/) were employed for statistical analysis. Kaplan-Meier method was used to calculate survival. Univariate Cox regression analysis was carried out to identify the variables significantly influencing survival (p<0.05). A multivariate Cox regression analysis was performed on all variables associated with survival to assess their independent association with survival. A statistical significance level of p<0.05 was set for these analyses.
Results
1. Patient demographics and clinical characteristics
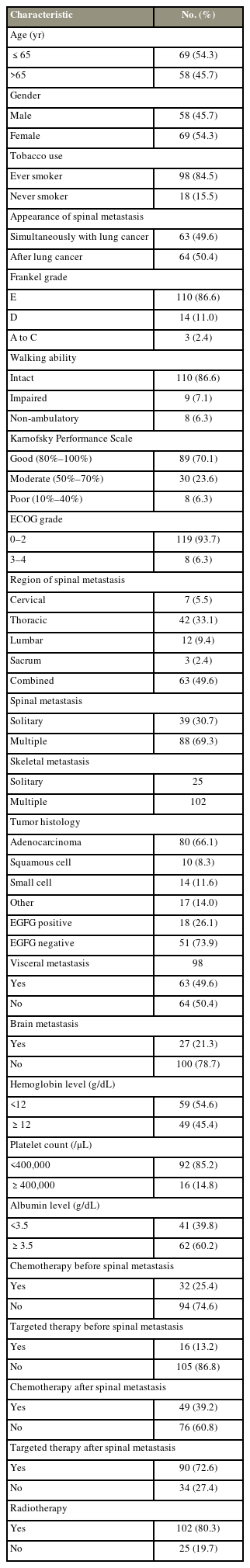
A total of 127 patients are included in this analysis. Patient data and treatment are summarized in Table 1. The median age is 64 years (95% confidence interval [CI], 63–67 years). The ratio of females to males is 1.2. Among the patients, 110 (86.6%) could walk normally. Eighty-nine patients (70.1%) had good KPS (80%–100%). Forty-two patients (33.1%) had thoracic spine metastatic, and 63 patients (49.6%) had metastases in more than one area of the spine. Eighty patients (66.1%) had adenocarcinoma. The median survival of the population research was 5.3 months (95% CI, 3.7–9.6 months). At 3 months, 6 months, and 12 months, the survival rates were 66.1%, 47.2%, and 33.1%, respectively (Fig. 1). The median follow-up was 5.13 months (95% CI, 3.3–8.8 months). Ten patients were still alive when the present study ended, and their median follow-up was 25.9 months.
2. Prognostic factors for survival
In the current study’s univariate analysis of 24 variables, patients with a high KPS score appeared to have a higher chance of survival (HR, 0.5; 95% CI, 0.24–1.04) but this did not reach statistical significance (p=0.06). Low hemoglobin was linked to shorter survival (HR, 1.49; 95% CI, 1.00–2.23; p=0.049), while targeted therapy after spinal metastasis was linked to longer survival (HR, 0.34; 95% CI, 0.21–0.51; p<0.001). Patients with low albumin seemed to have decreased survival (HR, 1.49; 95% CI, 0.99–2.25) but this did not reach statistical significance (p=0.06) (Table 2).
In the multivariate analysis, targeted therapy was independently associated with longer survival (HR, 0.3; 95% CI, 0.17–0.5; p<0.001). Patients with higher KPS appeared to live longer, but this did not reach statistical significance (HR, 0.46; 95% CI, 0.2–1.07; p=0.07). Patients with lower hemoglobin appeared to have a lower chance of survival, but this did not reach statistical significance (HR, 1.52; 95% CI, 0.98–2.35; p=0.059) (Table 3).
3. Predictive accuracy of six scoring systems
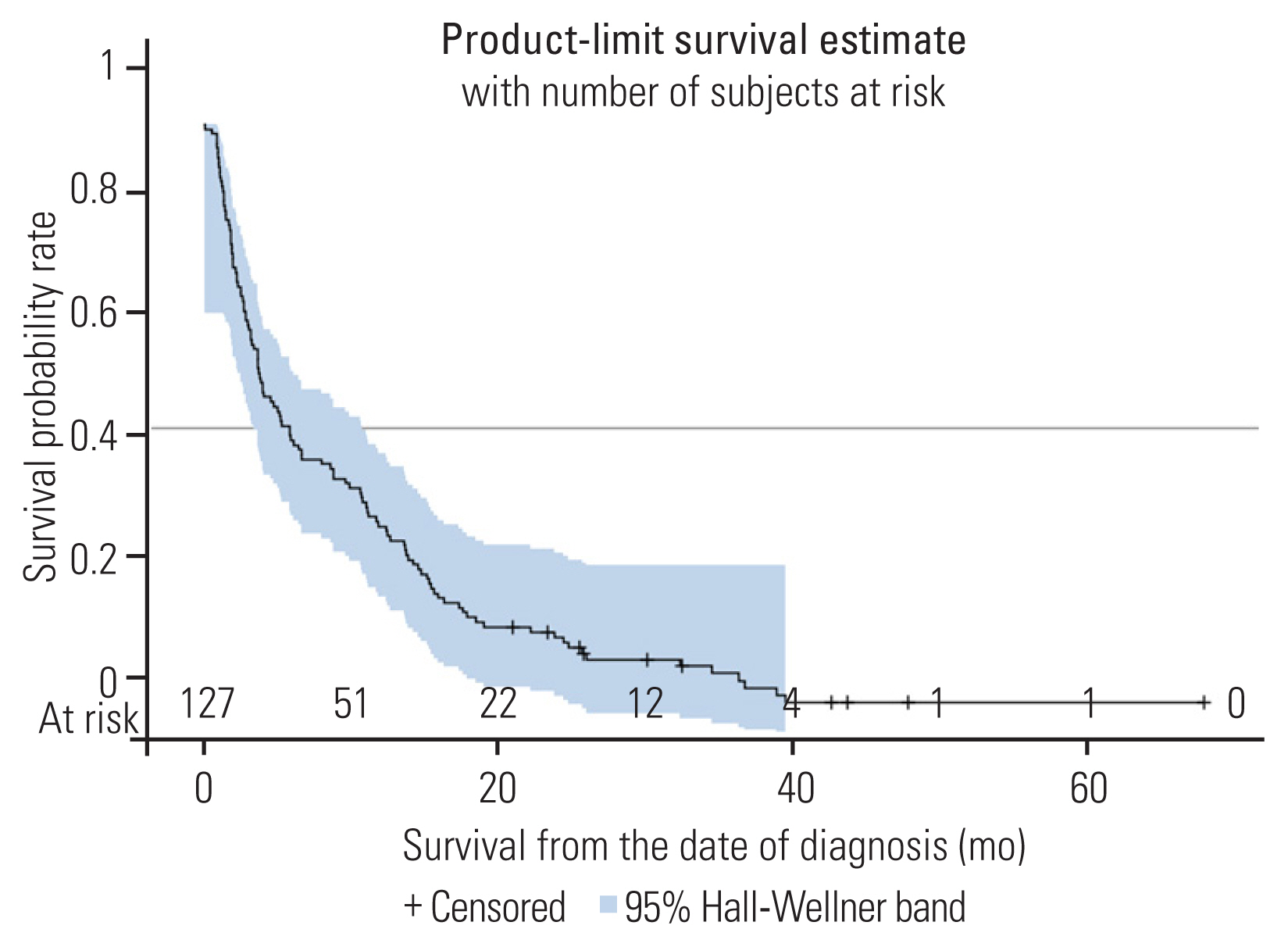
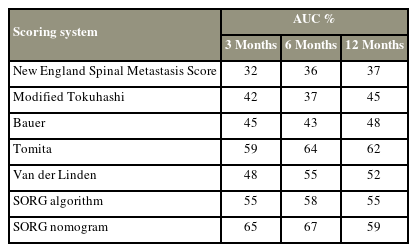
The AUC of the time-dependent ROC curves for the seven prognostic scores were calculated. In predicting 3-month survival, the SORG nomogram had the highest accuracy (AUC=0.65), followed by the Tomita score (AUC=0.59), and then the SORG algorithm (AUC=0.55) (Fig. 2). The SORG nomogram also predicted 6-month survival the best (AUC=0.67), followed by the Tomita score (AUC=0.64), and then the SORG algorithm (AUC=0.58) (Fig. 3). The Tomita score was the most accurate for a 12-month survival prognosis (AUC=0.62), followed by the SORG nomogram (AUC=0.59), and finally the SORG algorithm (AUC=0.55) (Fig. 4). Although the SORG nomogram came closest in predicting 6-month survival (AUC=0.67), the performance of all these seven prognostic scores was poor (AUC <0.7) (Table 4).

Receiver operating characteristic curve of the prognostic scores at 3 months. NESMS, New England Spinal Metastasis Score; SORG, Skeletal Oncology Research Group.
Receiver operating characteristic curve of the prognostic scores at 6 months. NESMS, New England Spinal Metastasis Score; SORG, Skeletal Oncology Research Group.

Receiver operating characteristic curve of the prognostic scores at 12 months. NESMS, New England Spinal Metastasis Score; SORG, Skeletal Oncology Research Group.
Discussion
1. Survival
The prognosis of patients with spinal metastasis from lung cancer is poor. The median survival of the present population study was 5.3 months (95% CI, 3.7–9.6 months). This is consistent with previous research, which found that median survival with lung-cancer-derived spinal metastasis ranged from 3.5 months to 14 months in surgical patients and 8.5 months in non-surgical patients [17–19].
2. Prognostic factors
Expected survival is an essential part of the treatment decision for lung cancer and spinal metastases. In the current study, univariate analysis of 24 variables revealed that low hemoglobin (<12 g/dL) was associated with worse survival, and targeted therapy after spinal metastasis was a favorable factor for prolonged survival. Multivariate analysis revealed that targeted therapy was independently associated with increased survival. In contrast, Uei and Tokuhashi [20] discovered that tumor histology, the KPS, paralysis state, and targeted therapy all had a significant impact on survival in a study of 207 lung cancer spinal metastasis patients, both surgical and non-surgical (univariate analysis). Multivariate analysis revealed the KPS and paralysis state independently influenced survival, whereas the targeted therapy did not [20]. Patients with good KPS appeared to have longer survival in the current study, but this did not reach statistical significance (HR, 0.46; 95% CI, 0.2–1.07; p=0.07).
The impact of neurologic status on survival is controversial. Tomita found that preoperative paralysis did not affect survival time [5]. Chen et al. [21] also found that the preoperative Frankel score had no significant impact on survival time. Similarly, univariate analysis in our study revealed that the Frankel score had no important effect on survival (p=0.16). In contrast, Truong et al. [19] reported that preoperative paralysis had an important effect on survival.
The impact of laboratory values on survival has been reported. In univariate analysis, low hemoglobin (<12 g/dL) was to be significantly associated with worse survival in the current study, which is consistent with the findings of Ahmed et al. [22] of 176 patients with spinal metastases from various primary cancers. However, in a univariate analysis of 86 renal carcinoma spinal metastases undergoing surgical treatment, Massaad et al. [23] found low hemoglobin (<12 g/dL) did not influence survival. A low albumin level has been linked to a shorter survival time in spinal metastases [24]. Patients with low albumin appeared to have shorter survival in the current study (HR, 1.49; 95% CI, 0.99–2.27), but this did not reach statistical significance (p=0.06).
Previous studies found a conflicting relationship between lung cancer cell type and survival. According to Aydinli et al. [25] lung cancer spinal metastasis with squamous cell carcinoma has a better prognosis than adenocarcinoma. Nonetheless, Uei and Tokuhashi [20] found that the adenocarcinoma group outlived the non-adenocarcinoma group. However, the present study shows that tumor histology has no significant impact on survival, which is concordant with the results of previous studies in non-small cell lung cancer spinal metastasis [18,26]. The differences in patient characteristics and treatment methods, including chemotherapy and targeted therapy, among the studies, in our opinion, may explain this discordance.
3. Accuracy of prognostic scores
The present study aimed to assess the accuracy of prognostic scores for non-surgical lung cancer spinal metastases. The Tomita score, revised Tokuhashi score, modified Bauer score, Van der Linden score, classic SORG algorithm, SORG nomogram, and NESMS were evaluated, with the SORG nomogram having the highest accuracy in predicting 3-month survival (AUC=0.65) and 6-month survival (AUC=0.67), and the Tomita score having the best performance in predicting 12-month survival (AUC=0.62). But the performance of all the scores was poor (AUC <0.7). This is consistent with the study by Tarabay et al. [27], which found that the revised Tokuhashi, revised Bauer, SORG classic, and NESM scores performed poorly in predicting the survival of lung cancer patients with spinal metastasis receiving surgical treatment. However, it is discordant with the outcome of past studies. For example, in a population of 176 metastatic spinal patients, Ahmed et al. [22] revealed the SORG Nomogram illustrated the highest accuracy at predicting 3-month survival (AUC >0.70) following surgery, and the original Tokuhashi was the most accurate at presuming 12-month survival (AUC=0.78). In a subgroup of 34 lung cancer, their study found that none of the SORG Classic Scoring Algorithm, SORG Nomogram, original Tokuhashi, revised Tokuhashi, Tomita, original Bauer, modified Bauer, Katagiri, and van der Linden scoring systems was sufficiently accurate at predicting 3-month survival after surgery (AUC <0.7), but the SORG Algorithm, SORG Nomogram, revised Tokuhashi, and van der Linden scores achieved sufficient accuracy at 12-month survival (AUC >0.70), of which the SORG Nomogram had the greatest accuracy (AUC=0.85) [22]. Paulino Pereira et al. [14] found that the SORG nomogram accurately predicted 3-month (AUC=0.74) and 12-month (AUC=0.78) survival in 100 patients undergoing surgery for metastatic spine disease, but the SORG, but the SORG classic algorithm did not (AUC <0.7). In a study of spinal metastatic renal cell carcinoma treated surgically, Massaad et al. [23] found that the SORG nomogram, SORG classic, original Tokuhashi, and original Bauer performed well (0.7< AUC <0.8), while the NESMS performed best (AUC >0.8). On the contrary, the present study showed NESMS had the poorest performance in predicting the survival of spinal metastasis lung cancer treated non-surgically at 3 months (AUC=0.32), 6 months (AUC=0.36), and 12 months (AUC=0.37). Previous research has found the NESMS to be useful in predicting survival with spinal metastases treated surgically as well as nonoperatively [16,28,29]. The differences between the current study and previous studies could be explained by a variety of factors. Different population characteristics, primary tumor heterogeneity, and treatment methods used in the various studies mentioned may explain the differences. Second, the seven scores evaluated were designed to predict patient survival with spinal metastasis undergoing surgery; and they may be ineffective in patients treated non-surgically. Finally, these scores might not apply to those with very poor survival due to lung cancer with spinal metastasis. To predict the survival of lung cancer patients with spinal metastasis treated nonoperatively, new prognostic scores will be required.
The present study has several limitations. To begin, the current study population was recruited at a single institution and may not be representative of patient populations in other settings. Further prospective multicenter studies are necessary to confirm the findings reported here. Second, the current study has the limitations of retrospective studies, such as missing, incomplete, or inaccurate data, as well as the possibility of incorrect data interpretation. Finally, patient data for this study were collected from 2008 to 2020, and over the course of 12 years, optimal treatment prognoses changed, which may not have been reflected in the prognosis scores we examined.
Conclusions
The seven scoring systems studied are not accurate in predicting the survival of patients with spinal metastasis from lung cancer treated non-surgically.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conception and design: VTT, ZW; administrative support: ZW; provision of study materials or patients: DS, ZW, SJY, DR, GLM; collection and assembly of data: VTT, FAS, TPYT, RD; data analysis and interpretation: FAS, VTT; manuscript writing: all authors; and final approval of the manuscript: all authors.