Complication Pattern of Sacral Primary Tumor Resection: A Study on the Risk Factors of Surgical Site Infection and Bowel or Bladder Dysfunction and Their Associations with Length of Hospital Stay
Article information
Abstract
Study Design
Retrospective open cohort study.
Purpose
The current study aimed to explore the pattern of complications after primary sacral tumor resection, to investigate the possible effect of several perioperative parameters on the development of complications, and to identify which complications are associated with the length of hospital stay (LOS).
Overview of Literature
Primary sacral tumor (pST) resection is associated with a high complication rate. However, the number of studies on these complications and their effect on LOS is limited.
Methods
The clinical data of 140 patients with pST surgeries and 106 subsequent patients with local recurrence surgeries in four subgroups (index surgery, local recurrence surgery, malignant, and benign tumor) were prospectively collected and analyzed. The prognostic value of several perioperative factors on the development of surgical site infection (SSI), bowel and bladder dysfunction (BBD), and LOS was investigated using the logistic and linear regression models.
Results
The overall complication rates were 61.2% after index surgeries and 50.9% after local recurrence surgeries. The most frequent complications were SSI, vegetative dysfunction, urinary tract infections, and neurological deterioration. Age >55 years, malignant tumors, and red blood cell transfusion had a predictive effect on the development of SSI in the logistic model (p<0.01, R2=0.43). Bilateral S2 or S3 resection commonly caused postoperative BBD (chi-square test=62.5, degrees of freedom=4, p<0.001). In the multiple linear regression model, wound dehiscence, BBD, systemic and urinary tract infection, cerebrospinal fluid leak, and neurologic deterioration were associated with a significantly long LOS (p<0.01, R2=0.62).
Conclusions
Surgical resection of pSTs has a high complication rate. Its common complications are SSI and BBD, both of which can have a significant influence on global therapeutic outcome. Malignant tumor diagnosis, old age, and red blood cell transfusion can remarkably increase the risk of SSI. Further, the development of BBD is significantly associated with the number of resected sacral nerve roots. By decreasing perioperative complications, LOS can decrease significantly.
Introduction
The surgical management of primary sacral tumors (pSTs) is challenging due to a complicated regional anatomy and limited experience attributed to the rarity of these pathologies [1,2]. pSTs are insidious and can have large volumes before diagnosis, thereby invading the sacral bone, nerve roots, retroperitoneal structures, and posterior soft-tissue elements. Due to the high ratio of aggressive benign (Enneking S3) and low-grade malignant (Enneking IA/B) lesions in this region, the management options are limited. To effectively treat these conditions, surgical resection, en-bloc resection in cases of malignant lesions, and even total sacrectomy in some cases are required [3]. Heavy particle radiotherapy is a promising alternative; however, owing to its lack of accessibility, it is not widely used [4]. The local recurrence rate after surgery, even in the case of tumor-free margins, is relatively high [5]. Further, surgical resection for local recurrence can be demanding. In both cases, the complication rate after pST surgery is high at 6%–75% [6]. However, only a few studies have reported the factors causing complications [7–10]. Similarly, some studies have evaluated the effect of complications on length of hospital stay (LOS) after sacral tumor surgeries [11–13], despite the fact that this is one of the most important inpatient indicators of surgical success.
The current study aimed to explore the complication pattern of pST surgeries in different patient subpopulations, to investigate the possible effect of several perioperative parameters on the development of perioperative complications, and to identify which complications are associated with the length of LOS.
Materials and Methods
1. Study design
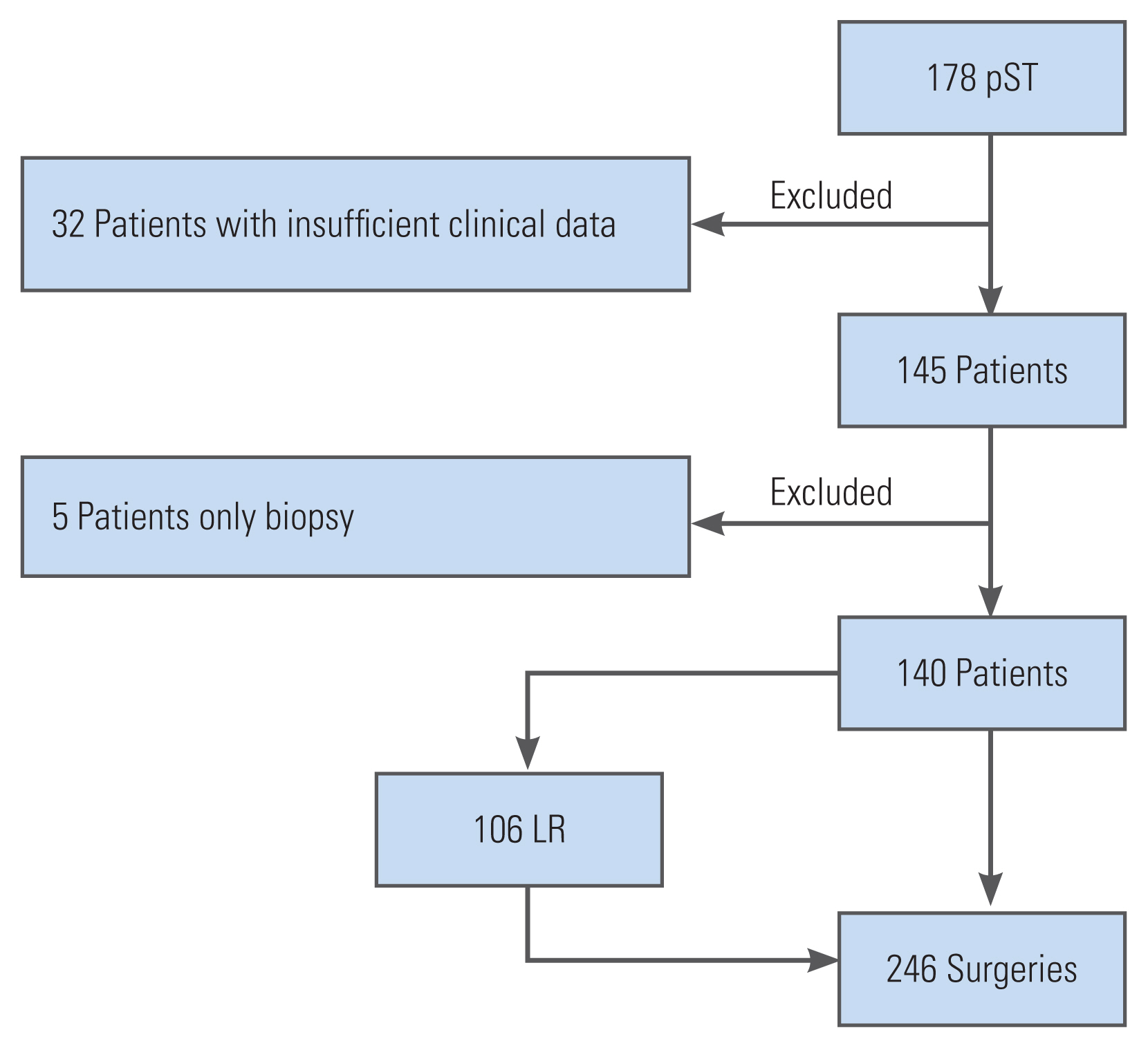
This retrospective open cohort study was conducted at an international spine tumor referral center. The inclusion criteria were patients diagnosed with pST who underwent surgical resection between January 1995 and December 2019 and those who developed local recurrences after the index surgeries who were treated surgically. The exclusion criteria were patients with secondary sacral tumors, lymphoma, or myeloma, those who only underwent biopsy, and those who had insufficient clinical data. Data collection was performed in a prospective, standardized manner (institutional tumor registry) (Fig. 1).
This study was approved by the Scientific and Research Ethics Committee of the National Medical Research Council (30851-2/2016/EKU). The need for patient informed consent was not required owing the nature of the study and the use of deidentified data.
2. Data collection
The following data were collected from the pre- and postoperative inpatient and outpatient clinical records: age, histological diagnosis, detailed medical history, body mass index (BMI), tumor volume, operating room (OR) time, surgical margins, use of implants, nerve resection, volume of blood loss, perioperative transfusion, length of intensive care unit stay, LOS, complications, and local recurrence. LOS was defined as the period between hospital admission and discharge to the patient’s home or physical rehabilitation care. The patients remained in the hospital until the treating physicians were certain that no additional surgical care was required to address early postoperative complications. All complications were treated by the same team.
The imaging (computed tomography scan and magnetic resonance imaging) results and histological diagnosis were used to determine localization and tumor volume. The height, width, and depth of the tumor were recorded. The volume was calculated using the formula for an ellipsoid mass (volume=π/6×height×width×depth) [6]. The detailed medical history of the patients was standardized using the Charlson comorbidity index.
In addition to categorizing resection (wide-marginal en-bloc resection versus intralesional surgery), the oncological outcome of surgery was determined via histopathologic examination (where R0 indicates tumor-free margins; R1, microscopic residuum; and R2, macroscopic tumor remnant). In case of total sacrectomy or if there was SI joint involvement, lumbopelvic reconstruction was performed using different constructs. Sacral resection was categorized according to the Fourney’s classification system [14]. In case of midline tumors, low sacral resection was performed at the S4 level and below, midsacral resection at the S3 level and below, and high sacral resection at the S2 level and below. Total sacrectomy was defined as bilateral resection at the S1–S5 levels. Eccentric lesions were resected via sacroiliac joint excision and hemisacrectomy. Nerve resection was categorized as no nerve resection or resection below the S3, bilateral S2, unilateral S2, bilateral S3, and unilateral S3 nerve roots. Perioperative transfusion was assessed based on the number of packed red blood cells (RBCs) transfused. All complications occurred intraoperatively and during the hospital stay and follow-up period. Follow-up data were obtained via either the direct examination of the patients or telephone interviews and data collection from electronic medical reports. Further, governmental vital statistics databases were accessed. The study data were collected and managed using the institutional REDCap electronic data capture system [15].
3. Statistical analysis
To enhance interpretability, data on index surgeries, local recurrence surgeries, and benign and malignant tumors were analyzed separately, thereby creating four subgroups: index surgery benign tumor (IB), index surgery malignant tumor (IM), local recurrence surgery benign tumor (LRB), and local recurrence malignant surgery (LRM). Index surgery was defined as the first surgery aiming at tumor resection. Biopsy or other previous diagnostic techniques were not considered. The differences between subgroups in the demographic analysis were analyzed using the chi-square test and analysis of variance with the Bonferroni post hoc test. The effects of several perioperative variables on the development of surgical site infection (SSI) were analyzed using univariate and multivariate logistic regression models. All variables identified previously in the literature and those with clinical plausibility were assessed using univariate analysis. A p-value of <0.1 was considered for a backward multiple regression model. The probability of logistic regression was used as a test variable in the receiver operating characteristic curve analysis to calculate the c-index of the model. The effect of nerve root resection on the development of bowel and bladder dysfunction (BBD) was analyzed using the chi-square test. A linear regression model was used to assess the effect of complications on LOS. The endpoint (LOS in days) was normalized using logarithmic transformation. All complications, other than mortality, cardiac arrest (which decreased the LOS), pressure sores (a complication of LOS), and dural tear (similar to cerebrospinal fluid [CSF] leak), were included in the analysis. Statistical analyses were performed using the IBM SPSS software ver. 27.0 (IBM Corp., Armonk, NY, USA).
Results
During the study period, 140 patients underwent primary sacral tumor resection. Most patients had a malignant sacral tumor (n=103, 73.5%) (Table 1). Local recurrence (LR) occurred after 46 index surgeries. In total, 106 LR surgeries were performed during this period.
1. Data on demographic and surgical characteristics
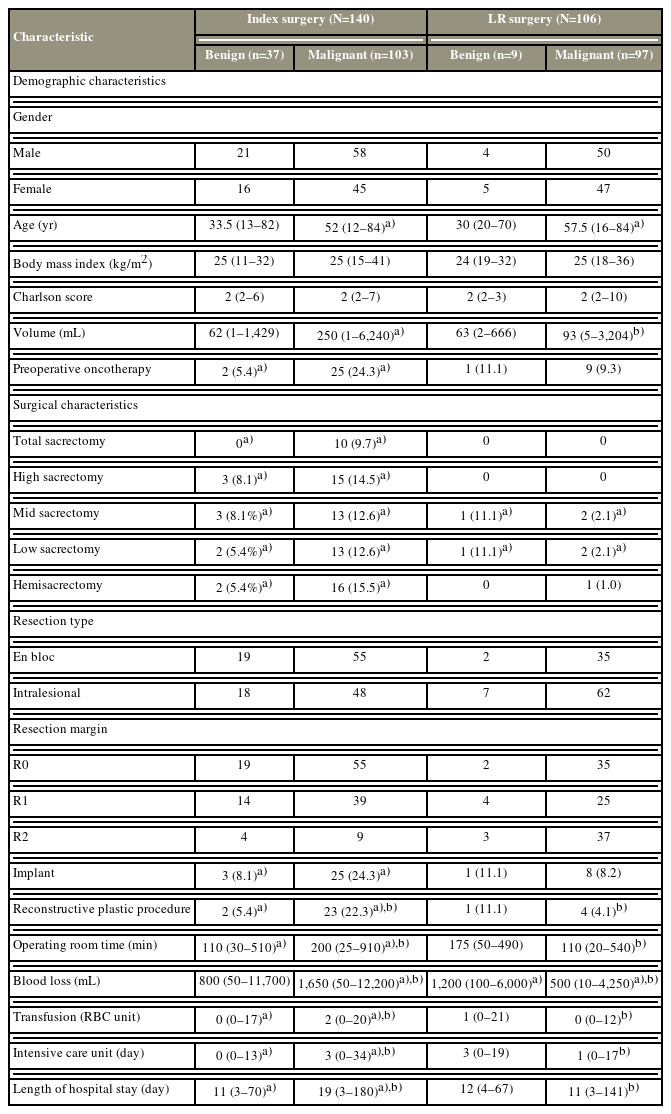
Table 2 shows the demographic and clinical characteristics of the four subgroups. The patients were homogeneously distributed in the subgroups according to sex, with a ratio of approximately 1:1. There was a statistically significant difference in terms of age distribution between malignant and benign cases, with a median age of 52 years and 57.5 years in the IM, LRM groups and 33.5 years and 30 years in the IB, LRB groups, respectively. The IM subgroup had the largest tumor volume, with a median of 250 mL (range, 1–6,240 mL). This value was significantly different from that of the IB and LRM groups. Similarly, the IM group (24.3%) commonly received neoadjuvant chemo- and radiotherapy compared with the IB group. Most patients were slightly overweight, with a median BMI of 25.0 kg/m2 and a relatively low median Charlson comorbidity index at 2.
In total, 10 sacrectomy procedures were performed in malignant cases (Table 2). The IM group commonly underwent wide resections. Meanwhile, the LRM group frequently had intralesional resection. Implants were used in 24.3% and 8.1% of malignant and benign index surgeries. Approximately 12.2% of surgeries involved a complex plastic closure, and there was a significant difference in the need for reconstructive plastic procedures between the IM, IB, and LRM groups. The IM group had a significantly high median volume of blood loss (1,650 mL), operating room time (200 minutes), and median number of pack RBCs transfused (2 packs). Similarly, among all groups, the IM group had the highest median length of intensive care unit stay and LOS (3 days and 19 days, respectively).
2. Complication rate
In our cohort, pST surgeries were characterized by a high overall complication rate at 56.5%. In total, 85 (61.2%) and 54 (50.9%) patients developed complications after index surgeries and LR surgeries, respectively. The number of complications per surgery ranged from 0 to 9, with at least one complication in 50 cases, 2 in 36 cases, 3 in 17 cases, 4 in 14 cases, and 5 in eight cases, and from 6 to 9 in 14 cases. Table 3 shows the distribution of complications among the IM, IB, LRM, and LRB groups. SSI and BBD were the most frequent complications, followed by a significantly high volume of blood loss and systemic infections. We lost 12 patients (4.8%) in the perioperative period (n=7 in the IM group and n=5 in the LRM group).
3. Surgical site infection
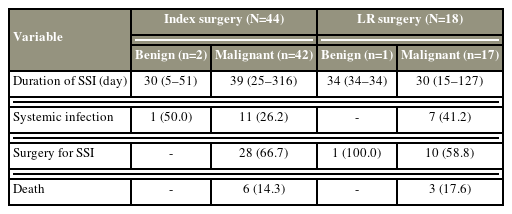
Wound complications were identified after 76 surgeries (30.9%) (wound dehiscence in 14 cases [5.7%] and SSI in 62 cases [25.2%]). SSI commonly occurred in the IM and LRM groups (42 [40.8%] versus 17 [17.5%]). Approximately 66.7% of patients in the IM group, 58.8% in the LRM group, and 100% in the LRB, SSI groups required one or more debridement surgeries. Systemic infection occurred due to SSI in 26.2% of patients in the IM group and 41.2% of patients in the LRM, SSI groups. Six patients died due to septic complications in the IM group and three in the LRM group (Table 4).
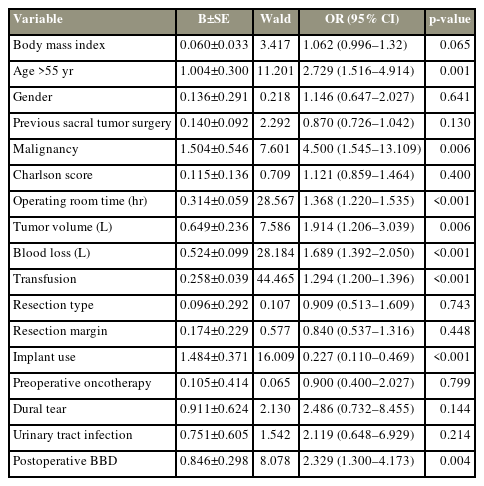
The effects of several perioperative variables (malignant/benign histology, BMI, age, previous sacral tumor surgery, Charlson comorbidity index, operating room time, tumor volume, volume of blood loss, transfusion, surgical margins, implant use, preoperative oncotherapy, dural tear, urinary tract infection, and postoperative BBD) on the development of SSI were analyzed using univariate logistic regression models (Table 5). Based on these factors, histological diagnosis, age, implant use, volume of blood loss, tumor volume, BMI, postoperative BBD, and transfusion were entered into a backward multiple logistic regression model. In the final model, age (odds ratio, 3.0), transfusion (odds ratio, 1.3), and malignant histology (odds ratio, 4.5) were significant risk factors for SSI (model: p<0.01, chi-square test=85.97, R2=0.43, c-index=0.863).
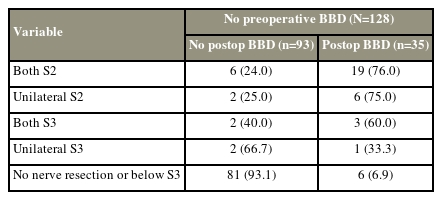
4. BBD
Of 140 index patients, 12 (8.5%) had preoperative BBD, and only two of them recovered after surgery. Among 128 patients without preoperative BBD symptoms before index surgery, 35 (27.3%) developed BBD after surgery (Table 6). The development of postoperative BBD was strongly associated with the level of nerve root resection (chi-square test=62.5, df=4, p<0.001). In total, 6 (6.9%) of 87 patients who had no nerve root resection or only below the S3 level developed postoperative BBD. Moreover, 25 patients underwent bilateral S2 resection, and only eight patients had unilateral S2 loss. In both groups, approximately 75% of patients developed postoperative BBD. The incidence rate of postoperative BBD in patients who underwent bilateral S3 resection was 60%, and unilateral S3 resection caused BBD in 33% of cases.
5. Length of hospital stay
The median LOSs were 14 (3–180) days in the whole cohort, 19 (3–180) days in the IM subgroup, and 11 (3–70) days in the IB subgroup (p<0.05). The median LOSs were 12 (4–67) days among patients who underwent local recurrence surgery in the LRB subgroup and 11 (1–141) days among those in the LRM subgroup. In the multiple linear regression model, SSI, wound dehiscence, BBD, systemic infection, urinary tract infection, CSF leak, and neurological deterioration had a significant effect on LOS. Approximately 62% of the total LOS variance was explained by the final mode (p<0.01, R2=0.62) (Table 7).
Discussion
As pSTs are rare and histologically heterogeneous, only a few studies have described the complication patterns of sacral tumor resections in different patient subpopulations, including those who underwent index versus LR surgeries and those with benign, malignant tumors. In our cohort comprising 140 patients with surgically treated pSTs and 106 subsequent patients with LR surgeries, the overall complication rates were 61.2% after index surgeries and 50.9% after local recurrences. The overall complication rate was higher in malignant pST surgeries in our cohort, which was consistent with the results of other studies. Angelini et al. showed that the complication rate can reach 80% in sacral chordoma resections [9]. However, it can be significantly low (17%) in patients with benign lesions [16].
Index surgeries are associated with a higher complication rate in our cohort. One of the reasons can be surgical invasiveness because wide en-bloc resection is the gold standard surgical therapy for malignant sacral tumors [1,3,6]. Further, there is a cautious follow-up protocol after index sacral tumor surgery at our institution. Hence, local recurrences are identified at an earlier phase. This notion is supported by data showing that the average tumor volume is smaller in cases of LR surgeries, which assumes less nerve, vessel involvement and smaller surgical wound, which can result in a lower risk of complications.
The most common complications were SSI and BBD. The incidence of SSI in our overall cohort was 25.2%, which is in the lower third of the published values (14.8%–44%) [6,8,12,17,18]. Malignancy was a significant predictor for developing SSI even in the multivariate model in our analysis and in other studies [8,19]. SSI and its predictors were analyzed in other studies that showed a high incidence rate but on a variable group of predictors. Li et al. [20] reported 29.2% of cases of wound-related complications in a large sacral tumor cohort. Results showed that previous irradiation, rectal injury, longer duration of surgery, and CFS leakage were significantly associated with SSI development. Chen et al. [21] conducted a retrospective analysis to assess the SSI rate after sacral chordoma resection. Of 45 patients, 16 (35.6%) developed postoperative wound infections. Significant risk factors were low albumin levels (<3.0 g/dL), long surgery duration (>6 hours), and previous history of surgery. Similarly, Sciubba et al. [17] reported an SSI rate of 39% in 46 patients with surgically treated malignant sacral tumors. In their cohort, previous history of surgery, number of surgeons involved in the surgery, old age, presence of complex soft-tissue reconstruction, and BBD were associated with a higher risk of SSI. In contrast, Marmouset et al. [22] did not find an association between bowel incontinence and SSI after en-bloc sacrectomy. In our cohort, BBD was associated with SSI in the univariate analysis. However, it lost its significance in the multivariate model. SSI after spinal surgical procedures is generally more frequent in elderly individuals [22–24], which is in accordance with our results, where age >55 years has resulted in a 3-fold risk of having an SSI after sPT surgeries. sPT resection is commonly characterized by a significant volume of blood loss, and patients often require RBC transfusion during the intraoperative and/or postoperative periods. A previous study showed the immunosuppressive effect of homologous blood transfusion [25]. De la Garza Ramos et al. [26] reported that transfusion after metastatic spinal tumor surgery significantly increased the overall complication rate. Results showed a similar prognostic effect of transfusion on the development of SSI in our pST cohort, and the association of which remained significant in the multivariate model. Nakai et al. [27] emphasized the role of autologous blood transfusion in sacral tumor resection, which is the mechanism underlying the association.
The incidence of BBD (16.2%) in this research is lower than that in other studies, which is 38%–78% depending on the composition of the study cohort [6,8,12,17,18]. The results of published data about the association between the level of nerve root resections and the risk for BBD vary. Our results support the findings of Fourney et al. [14] who reported that patients with amputations distal to the S3 levels only generally experienced minor or no deficit, with the preservation of sphincter function in most cases. The S2 roots are often considered vital for bowel, urinary, and sexual functions, and several authors have published that the preservation of the bilateral S2 roots decreases the chance of BBD. However, a significant group of patients developed BBD after unilateral S2 or bilateral S3 root resections. This result is not in accordance with previous findings [28,29]. However, it supports the results of a systematic review conducted by Zoccali et al. [28]. Accordingly, in a case series of 50 sacrectomy procedures, Guo et al. [12] found a close association between resection of the S3 nerve roots and the development of BBD. Our study supports their findings. However, we must emphasize the fact that even unilateral S3 resection can lead to BBD in a third of these cases.
LOS is among the few parameters widely used to characterize the complexity, severity, and economic consequences of a medical condition or treatment. However, only few papers have published this parameter in case of pST surgeries. The median LOS in our study was shorter than that of other publications. Moreover, SSI, wound dehiscence, BBD, systemic and urinary tract infections, CSF leak, and neurologic deterioration had a prolonged effect on LOS after sacral tumor resections. In case of 75 index (53.5%) and 32 LR (30.2%) surgeries, at least one of these LOS prolonging complications occurred. Guo et al. [12] found that the median LOSs after sacrectomy procedures were 48.5 days in case of colostomy and 18.5 days in participants without colostomy. Moreover, patients who underwent surgery using a myocutaneous flap and those with BBD had a longer LOS [12]. Feghali et al. [13] showed that iliac vessel involvement, larger tumor volume, staged procedure, and S1 nerve root sacrifice were independent predictors of prolonged LOS after sacral tumor surgery.
The current study had some strengths and limitations. In terms of limitations, previous studies on this topic are related to the heterogeneous histological distribution of tumors and different treatment principles according to the Enneking’s criteria. Further, the data collection had a relatively long-time frame. However, the principles of surgical treatment for pSTs have not changed during this period. Finally, an external model validation was not performed. Regarding the study’s strengths, a relatively large patient population was included, and prospectively collected data were mainly analyzed. The high number of cases allowed us to analyze the effect of tumor dignity on the complication pattern, thereby emphasizing that a malignant histology is an independent risk factor of SSI in pSTs. Nevertheless, further studies on the risk factors of various complications should be performed using a large multicentric dataset by incorporating other important outcome variables, such as quality of life.
Conclusions
To the best of our knowledge, to date, this is the largest study focusing on complication patterns after primary sacral tumor surgeries. The complication rates were high at 61.2% after index surgeries and 50.9% after LR surgeries. Malignant tumor diagnosis, age >55 years, and number of pack RBCs transfused had a predictive effect on the emergence of SSI, which is among the most common types of complications. Bilateral S2 or S3 nerve root resection commonly resulted in postoperative BBD. However, this severe complication was also frequently observed in unilateral S2 resection and occurred in cases of unilateral S3 nerve root resection. SSI, wound dehiscence, BBD, systemic and urinary tract infection, CSF leak, and neurological deterioration are associated with an increased risk of prolonged LOS. Based on our findings, SSI and BBD are the most common complications of pST surgeries. Moreover, malignant tumor diagnosis, old age, and RBC transfusion remarkably increased the risk of SSI, and LOS can decrease significantly by decreasing perioperative complications.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by KK. The first draft of the manuscript was written by KK, PPV, and ZS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript. All the authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This work was supported by the UNKP-21-5 New National Excellence Program of the Ministry for Innovation and Technology from the source of the National Research, Development, and Innovation Fund as well as by the by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences.