An Algorithmic Roadmap for the Surgical Management of Degenerative Cervical Myelopathy: A Narrative Review
Article information
Abstract
Degenerative cervical myelopathy (DCM) is a leading cause of disability, and its surgical management is crucial for improving patient neurological outcomes. Given the varied presentations and severities of DCM, treatment options are diverse. Surgeons often face challenges in selecting the most appropriate surgical approach because there is no universally correct answer. This narrative review aimed to aid the decision-making process in treating DCM by presenting a structured treatment algorithm. The authors categorized surgical scenarios based on an algorithm, outlining suitable treatment methods for each case. Four primary scenarios were identified based on the number of levels requiring surgery and K-line status: (1) K-line (+) and ≤3 levels, (2) K-line (+) and ≥3 levels, (3) K-line (−) and ≤3 levels, and (4) K-line (−) and ≥3 levels. This categorization aids in determining the appropriateness of anterior or posterior approaches and the necessity for fusion, considering the surgical level and K-line status. The complexity of surgical situations and diversity of treatment methods for DCM can be effectively managed using an algorithmic approach. Furthermore, surgical techniques that minimize the stages and address challenging conditions could enhance treatment outcomes in DCM.
Introduction
Degenerative cervical myelopathy (DCM), a prevalent neurological disorder, exhibits varying prevalence and incidence rates because of the diverse classifications of degenerative processes and pathologies [1]. It is considered the most common cause of spinal cord dysfunction among adults, with a recent study estimating the prevalence of related hospitalizations of 4.04 per 100,000 person-years [1,2]. The pathophysiology of DCM is multifactorial. DCM encompasses various degenerative conditions, including spondylosis, degenerative disk disease, ossification of the ligamentum favum, and ossification of the posterior longitudinal ligament (OPLL) [3]. Cervical spondylosis is an age-associated degeneration of the cervical spine caused by excessive motion and repetitive trauma. Spondylosis begins with degenerative changes in the disk space and adjacent structures, including facet joints, uncovertebral joints, posterior longitudinal ligament, and the ligamentum flavum [1]. OPLL can also cause spinal cord compression, resulting in myelopathy. OPLL is more common in Asian populations. Computed tomography (CT) revealed that the incidence of OPLL was as high as 6.3% in the Japanese populations compared with 2.2% in non-Asian populations [4,5]. Histologically, OPLL is caused by fibroblast and chondroblast proliferation and intramembranous ossification [6].
Surgical treatment aims for adequate decompression of the spinal cord, restoration of cervical alignment, and fusion, if instability is present [7,8]. Various surgical approaches have been developed, and controversy still exists regarding which operation should be undertaken for different preoperative conditions. Thus, surgical planning tailored to each patient’s situation is now becoming more important. This review addresses surgical strategies for cervical myelopathy based on existing preoperative evaluation systems and techniques.
Strategic Surgical Approaches for Degenerative Cervical Myelopathy: Evaluating Anterior, Posterior, and Combined Methods
For DCM, various surgical options exist, and the optimal approach should be tailored to each patient’s specific characteristics. Understanding the advantages and disadvantages of each surgical technique is crucial to achieve the best outcomes in patients with DCM. The anterior approach offers several benefits over the posterior approach: it allows direct decompression of ventral pathologies (such as disk disease, bony spurs, and OPLL), often resulting in minimal neck pain, and aids in the restoration of cervical alignment [9,10]. Spine surgeons typically prefer the anterior approach for cases involving 1–2 levels, as complication rates tend to increase in patients with disease of ≥3 levels [11–13]. Conversely, the posterior approach is suited for longer-level decompression. Its effectiveness hinges on the spinal cord’s ability to shift posteriorly following decompression. However, in cases of kyphosis greater than 10°–13°, neurological recovery may not be as predictable because a posterior drift of the cord is less likely to occur [14,15].
The shape and size of the mass caused by OPLL or spondylosis can also affect the outcome. The canal-occupying ratio represents the proportion of the spinal canal occupied by the mass [16]. Notably, when this ratio exceeds 60%, the neurological outcomes following posterior decompression tend to be less favorable than those following anterior decompression [17]. This is particularly the case when the mass protrudes sharply, potentially diminishing the expected degree of neurological recovery [17]. Therefore, two critical prerequisites for effective posterior decompression would be a kyphosis of <10° in cervical alignment and an adequate anteroposterior diameter of the spinal canal. Fujiyoshi et al. [18] introduced the concept of the K-line to consider both the shape of the lesion and cervical alignment in patients with DCM. The K-line is defined as a straight line connecting the midpoints of the spinal canal at C2 and C7 on lateral cervical radiographs and helps in categorizing patients with DCM into those whose pathologies lie anterior to the kyphosis line (K-line positive) and those that cross posterior to the kyphosis line (K-line [−]). Surgeons frequently use the K-line to determine the most suitable surgical approach. Recently, various detailed preoperative evaluation modalities stemming from the K-line, such as the modified K-line [19] and kappa-line [20] have been developed. However, many other parameters must be considered to achieve the best results for any patient, and choosing the optimal treatment is often difficult. Consequently, choosing between the anterior versus posterior approach remains challenging, particularly when considering sagittal alignment, number of levels involved, and lesion morphology and type. In cases where rigid kyphosis and posterior pathologies coexist, a combined anterior and posterior approach may be necessary. A comprehensive understanding of the advantages and disadvantages of each surgical approach is crucial to achieve the best possible outcomes in patients with DCM (Fig. 1). To aid in this process, we propose some guidelines based on our experience, presented as a treatment algorithm (Fig. 2). This approach aims to guide clinicians in making informed decisions tailored to the unique needs of each patient with DCM. Of course, surgeons should choose the approach that they are most comfortable with and that works best in their hands [21]. Prospective, multicenter studies have reported that neither the anterior nor the posterior method can be declared definitively superior because each technique may be advantageous in various situations [21,22]. This study aimed to outline the general advantages and disadvantages of each approach, as supported by current evidence, and offer possible options to serve as a guide for typical scenarios. Our goal is not to dictate a specific course of action but to assist in informed decision-making that aligns with the best interests of each patient.

Surgical options for degenerative cervical myelopathy including the anterior approach and the posterior approach. DCM, degenerative cervical myelopathy; ACDF, anterior cervical discectomy and fusion; VBSO, vertebral body sliding osteotomy.
Treatment Algorithms
1. K-line positive (compressive pathology lies ventral to the line) and ≤3 levels
Fig. 3 illustrates a case of cervical compressive myelopathy resulting from a disk protruding at C4–C5, and migrating upward either a C4–C5 diskectomy or a C4 corpectomy is often selected for several reasons: (1) the pathology, located anterior to the cord, can be more readily accessed and removed via an anterior approach, and (2) the anterior approach reduces the number of surgical levels involved. While the posterior approach would typically necessitate including at least the laminas of C3–C5, anterior decompression would involve only one or two levels; and (3) the loss of range of motion through anterior fusion is generally insignificant within a two-segment operation, and the risk of nonunion is relatively low [23]. Thus, the anterior approach is better for lesions with ≤3 levels (Fig. 2).
The choice of treatment for cervical compressive myelopathy depends on the lesion’s location and shape, with various anterior approaches including anterior cervical discectomy and fusion (ACDF), corpectomy, and vertebral body sliding osteotomy (VBSO) being options (Fig. 1). However, these are not absolute indications. Even in cases involving <2 levels, posterior surgery may be appropriate, depending on the lesion’s location and the surgeon’s familiarity [24]. A recent meta-analysis reported difficulty in determining the optimal surgical approach for treating multilevel DCM [25]. For diseases involving ≤3 levels, the posterior approach is less favorable than the anterior approach, particularly when dealing with anterior spinal cord pathologies because of limitations in achieving indirect decompression [26]. ACDF is often considered the standard treatment for 1–2 level diseases [12,27–31]. Another alternative is corpectomy, which reduces the number of graft–host interfaces and allows for decompression behind the vertebral body [12,28,29,32,33]. However, corpectomy tends to present more perioperative challenges than ACDF. In a systematic review of 1,372 patients, Han et al. [34] found that ACDF yielded better outcomes than corpectomy in terms of complications and cervical sagittal alignment.
To address these concerns, we previously reported the use of the novel VBSO technique as an alternative to corpectomy for treating DCM [35]. VBSO involves anteriorly translating the vertebral body to expand the spinal canal, thereby reducing the need for direct removal of pathologies such as ossified masses and bony spurs [6,31,33,36]. Compared with corpectomy, VBSO has fewer complications because it minimizes the direct detachment of the pathology from the dura [37]. In summary, the optimal strategy should involve weighing the advantages and disadvantages of different approaches to create a tailored plan for each patient.
2. K-line (+) and >3 levels
In patients with K-line (+) status and lesions involving >3 levels, the posterior approach may be preferable for most surgeons. This is due to the increased risk of complications associated with the anterior approach in such cases [19,25,26,38–40]. Anterior decompression, particularly in cases involving >3 levels, significantly raises the risk of complications such as dysphagia, pseudarthrosis, and graft failure [30,41–45]. In addition, complications from corpectomy tend to be more severe, with increased risks of graft dislodgement and metal failure when ≥2 vertebral bodies are sacrificed [13,32]. Sasso et al. [12] reported a high graft failure rate and a substantial need for revision surgery following multilevel corpectomy. Therefore, when decompression of >3 levels is required, either a posterior decompression or a circumferential approach, rather than the anterior approach, must be considered (Fig. 2).
Effective posterior decompression hinges on two critical conditions [46]. First, following the lamina removal or expansion, the spinal cord must have sufficient space to drift posteriorly. Second, a lordotic sagittal alignment must be maintained; failure can result in a bowstring effect on the cord because of kyphosis [47]. To facilitate this posterior drift of the cord, the surgical procedure should be extensive enough to include both the upper and lower levels of the pathology. If this is not achieved, the cord may become trapped at the upper or lower level, leading to further neurologic compression. Studies on the efficacy of posterior decompression in relation to the degree of kyphosis have shown that when kyphosis exceeds 10°–13°, neurological recovery is less likely due to inadequate posterior drift of the cord [14,15,48]. Uchida et al. [15] reported that patients with DCM and either kyphosis or sigmoid alignment had better postoperative neurological outcomes with an anterior approach, as measured by the Japanese Orthopaedic Association score. For patients with marked kyphosis, either an anterior approach or surgical correction is recommended [31].
In the K-line (+) group and lesions involving >3 levels, the posterior approach is generally recommended as the initial surgical option (Fig. 2). Two surgical options are considered in this context: laminectomy with fusion and laminoplasty [19,20,49,50]. The key difference between these methods lies in whether fusion is performed. The neurological outcomes of laminoplasty and laminectomy with fusion for myelopathy are not significantly different [29,48,51,52]. Therefore, laminectomy with fusion is advised in patients presenting with confirmed neck pain and instability. Conversely, in cases where neck pain is absent or mild, laminoplasty is the preferred option because of its decreased complication rates and cost-effectiveness—it is approximately 2.3 times less expensive than laminectomy with fusion [52–54]. This was demonstrated in a recent prospective, randomized, multicenter study by Ghogawala et al. [55], comparing anterior and posterior approaches for myelopathy. All potentially randomizable cases were presented to the authors, and the majority had to agree that either an anterior or posterior procedure was reasonable. Then, the patients were randomized to either anterior or posterior surgery. If randomized to the anterior approach, the surgeon could choose either ACDF or corpectomy. If randomized to the posterior approach, the surgeon could choose either laminoplasty or laminectomy and fusion. Laminoplasty had the lowest complication rates of any procedure [55]. However, performing laminoplasty in patients who already suffer from axial neck pain or instability might exacerbate these conditions, potentially leading to worsened neck pain, progressive deformity, and neurological decline [56–58].
Overall sagittal alignment is another consideration in choosing a patient for posterior surgery. If laminoplasty is performed in patients with an increased C2–C7 sagittal vertical axis greater than 35–40 mm, the probability of developing postoperative deformity and neck pain is higher. In addition, a higher T1 slope (>20°) increases the risk of postoperative kyphosis and may affect outcomes. Therefore, in such patients, alternative methods to correct the malalignment may be necessary [57,59–63].
3. K-line (−): compressive pathology extending posterior to the line
The neurological results tend to be less favorable when posterior decompression is performed in the K-line (−) group [50]. Although the outcomes may improve relative to the presurgical condition, the risk of various complications of laminoplasty increased, which would hinder the achievement of a full or satisfactory neurologic recovery [64]. In addition, posterior decompression in the K-line (−) group is linked with a higher likelihood of transient C5 palsy, postoperative kyphotic deformity, worsening of neck pain, and progression of pathologies such as OPLL [65–68]. Therefore, alternative methods should be considered for the K-line (−) group. These include anterior decompression and posterior decompression, with a focus on changing the K-line status. Anterior decompression and fusion not only decompresses the spinal cord but also has the potential to alter the K-line from (−) to (+). This alteration is achieved through the restoration of cervical lordosis and removal of the pathology. If laminectomy and fusion is considered the posterior approach, the alignment must be restored such that all of the compressive pathology lies ventral to the K-line. Even with a total laminectomy, the posterior drift of the cord is inadequate to achieve a full decompression if the compressive pathology lies dorsal to the K-line.
4. K-line (−) and ≤3 levels
The anterior approach usually leads to better outcomes than posterior surgery in the K-line (−) group because anterior surgery includes direct resection of the anterior pathology and restoration of the cervical alignment [29,39]. If the pathology is located behind the vertebral body, corpectomy is a conventional surgical method used in the anterior approach [28,69]. However, corpectomy can be technically challenging for some surgeons and is associated with a higher incidence of surgery-related complications. Previous research has highlighted the elevated risk of intraoperative or postoperative implant dislodgement and dural tears during corpectomy [69–71]. In addition, cerebrospinal fluid (CSF) leakage is a notable complication of corpectomy, potentially leading to significant increases in operating time [39,70,72]. The floating island method for OPLL is considered when the risk of CSF leakage is high, particularly when accompanied by a double-layer sign in CT [67,72]. This technique involves thinning down the OPLL, leaving an island of the ossified dura intact, and letting it float away from the cord [73]. Despite being effective in decreasing the risk of dural tears, it has drawbacks, such as potentially insufficient decompression versus dural violation [67].
VBSO allows for the expansion of the spinal canal by anteriorly translating the involved vertebral bodies (Fig. 4). Previous studies have demonstrated that VBSO has advantages over corpectomy, notably in reducing the risks of dural tears and pseudarthrosis while effectively decompressing the spinal cord [36,37,49,74]. In addition to providing adequate decompression, VBSO has shown demonstrated results in restoring cervical lordosis and improving sagittal alignment (Fig. 5) [31,36]. It also achieves faster union than ACDF [33,49]. Recent studies have indicated that VBSO exhibits a higher fusion rate across various parameters, such as interspinous motion, intragraft bone bridging, and extragraft bone bridging and shows a markedly lower subsidence rate than corpectomy [33]. Pseudarthrosis and subsidence following anterior cervical surgery can lead to poor clinical outcomes, such as persistent neck pain and radiculopathy, and may necessitate revision surgery [65,75]. The rapid and robust fusion following VBSO is attributed to the ample exposure of the bone marrow facilitated by lateral troughs [33]. The multiple fixation points in VBSO, as opposed to the long-strut single graft in corpectomy, create a shorter and more stable lever-arm effect [32,33]. This stability contributes to a lower subsidence rate and reduced likelihood of kyphotic changes postoperatively [31,36,74,76]. The dramatic restoration of cervical lordosis after VBSO is further enhanced by the insertion of multiple lordotic cages. Therefore, VBSO is recommended as an alternative to corpectomy, particularly for patients with a high canal space-occupying ratio and K-line (−), owing to its effectiveness in reducing complications and enhancing surgical outcomes [36,37,74,76].

Procedure of vertebral body sliding osteotomy. (A) Two parallel longitudinal slits are made along the medial borders of the uncinated process. (B) Anterior translation of vertebral body is done by Allis forceps. (C) Cervical interbody cages are inserted with Caspar retractor distracted. (D) Cervical column is temporarily stabilizaed by releasing the traction force made by a Caspar retractor. (E) The protruding protions of the anteriorly translated bodies are shaved down using a high-speed burr or Leksell rongeurs. (F) Anteiror plating is done with plate and screw.

A 59-year-old woman with cervical spondylotic myelopathy due to rheumatoid arthritis and cervical kyphosis who underwent anterior decompression and fusion including vertebral body sliding osteotomy (VBSO) at C4 and C5. (A) Preoperative C3–6 cervical angle was 24° of kyphosis. (B) After VBSO, the C36 cervical angle was 10° of lordosis.
5. K-line (−) and >3 levels
In cases involving >3 levels (Fig. 2), posterior decompression is often preferred because of the increased risk of complications such as pseudarthrosis following extensive anterior surgery [25]. However, for K-line (−) cases, simply performing posterior decompression might not suffice for adequate neurological recovery; thus, altering the K-line from (−) to (+) becomes essential. This can be achieved by restoring cervical lordosis, which facilitates a posterior drift of the spinal cord. The restoration of the lordotic curve alters the K-line status because it can be adjusted surgically [77]. If the kyphosis is flexible, the posterior-only approach can be used [24,31]. For assessing flexible kyphosis, obtaining a plain lateral flexion–extension dynamic radiograph is crucial. If the cervical alignment is neutral to lordosis in the extension lateral radiograph, the patient may be categorized as having DCM with flexible kyphosis [78,79]. In such cases, posterior decompression through laminectomy and fusion, performed in the neck extension position, can be a viable and safe option. This approach not only ensures adequate decompression but also facilitates K-line alteration [31]. Conversely, if the kyphosis is rigid, a posterior-only approach could exacerbate the kyphosis because of the disruption of the posterior tension band, increasing the risk of screw pull-out. In cases where rigid kyphosis persists and adequate lordosis is not achieved even in the neck extension position, surgical intervention to restore cervical lordosis becomes necessary for K-line alteration [31]. In lordosis, the foramen closes down further than when the cervical spine is in a neutral or kyphotic position. Therefore, if a patient has foraminal stenosis and the neck is in a lordotic position and rigidly fixed, iatrogenic root deficits may occur. This has been demonstrated in numerous studies, where a laminectomy and fusion resulted in C5 and C8 palsies and recently verified in the study by Ghogawala et al. [55], which noted 12% major and 3% minor motor root deficits following laminectomy and fusion.
To convert a K-line from (−) to (+) in cases of rigid kyphosis, the inclusion of an anterior approach is recommended. With an anterior-first approach, one can decompress the foramina and restore the interpedicular height and lordosis. Then, posterior stabilization and fusion is performed to maintain alignment and ensure a high fusion rate. Others prefer to perform posterior decompression and screw fixation first, followed by anterior surgery to alter cervical alignment, and then, they apply a posterior rod [80,81]. This sequence of procedures, known as the circumferential or combined approach (P-A-P surgery), is relevant for patients with instability, severe osteoporosis, or a high risk of graft failure [81,82]. To streamline the circumferential approach, research has focused on making the three stages of P-A-P surgery more efficient. VBSO has been instrumental in this regard by reducing the number of surgical stages [31,36,76]. For instance, Fig. 6 shows a patient with K-line (−) DCM and staircase deformity, who had severe osteoporosis (T-score=−3.6) caused by the prolonged use of steroids for rheumatoid arthritis. Although the standard treatment in such cases of rigid kyphosis would typically be the circumferential P-A-P surgery [79], VBSO was employed instead. This approach successfully achieved both spinal cord decompression and K-line alteration. Considering the patient’s severe osteoporosis, posterior screw fixation without decompression was also performed on the same day. VBSO proved advantageous by accomplishing cervical alignment restoration and adequate decompression in just one stage of anterior surgery, without the common risks associated with corpectomy, such as dural tears and CSF leakage (Fig. 7). Thus, in patients indicated for P-A-P surgeries, VBSO can reduce the surgical stages to a single session. This technique has gained recognition for its distinct advantages over traditional methods in treating patients with DCM [24,83].
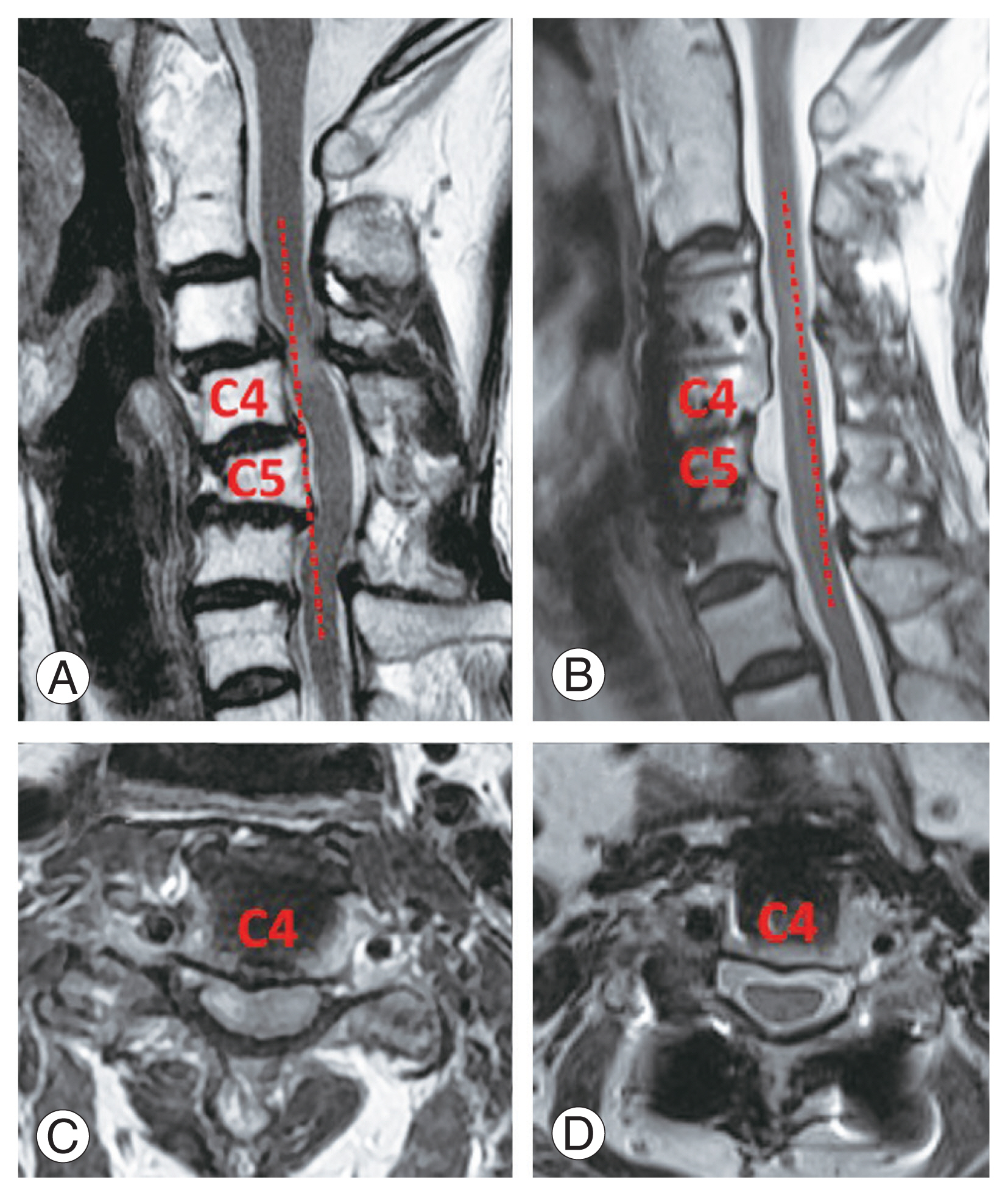
Preoperative and postoperative computed tomography and magnetic resonance imaging images for the 59-year-old woman in Fig. 5 undergoing vertebral body sliding osteotomy (VBSO) C3–6. (A, B) The K-line was negative preoperatively and positive postoperatively. (C, D) Adequate decompression was achieved after a single stage of VBSO.

Preoperative and postoperative magnetic resonance imaging images of a 67-year-old woman undergoing vertebral body sliding osteotomy (VBSO) C5–6. Sagittal image (A, B) and axial image at C5–6, C6, and C6–7 (C–H). A double-layer sign was observed in the postoperative axial image at C5–6 (red arrow). Adequate anterior decompression was achieved after VBSO.
A solution for addressing cervical myelopathy caused by continuous OPLL was previously proposed [84]. We have highlighted that K-line (−) DCM cases typically yield poor outcomes if treated solely with posterior decompressive surgery. In these cases, corpectomy is often considered, particularly when dealing with continuous or mixed-type OPLL located behind the vertebral body. To mitigate these risks, we introduced the greenstick fracture technique to reduce complication rates while restoring cervical lordosis [84]. This technique involves initial posterior decompression, followed by thinning of the OPLL mass using bur drilling after diskectomy. Instead of completely excising the OPLL mass, an incomplete fracture is created in the shallow mass, and a lordotic cage is inserted to restore cervical lordosis. This technique is presented in Fig. 8. A sagittal magnetic resonance image reveals severe cord compression, with a confirmed K-line (−) status. A kyphotic alignment is also observed in the plain lateral radiograph (Fig. 8E). CT indicates the presence of continuous OPLL (Fig. 8C), suggesting that this patient experiences cord compression across >3 levels and presents with rigid kyphosis, categorizing them as a K-line (−) case. Consequently, both K-line alteration and decompression are essential requirements for the surgeon (Fig. 2). In this scenario, posterior decompression with the laminoplasty technique (Fig. 8F) was initially selected. The presence of continuous OPLL and involvement of >3 levels made decompression and K-line alteration through VBSO technically challenging. Consequently, decompression was achieved through laminoplasty, followed by a three-level ACDF performed anteriorly. The greenstick fracture technique was applied to restore cervical lordosis, thus achieving a simpler two-stage P-A surgery instead of the more complex three-stage P-A-P procedure. Notably, the presence of continuous OPLL provided an advantageous aspect regarding instability, allowing us to substitute posterior fusion with laminoplasty. Both VBSO and the greenstick fracture technique are centered on the concept of K-line alteration. However, VBSO also facilitates canal expansion, which eliminates the need for initial posterior decompression that might be necessary to prevent canal narrowing caused by changes in lordotic alignment.
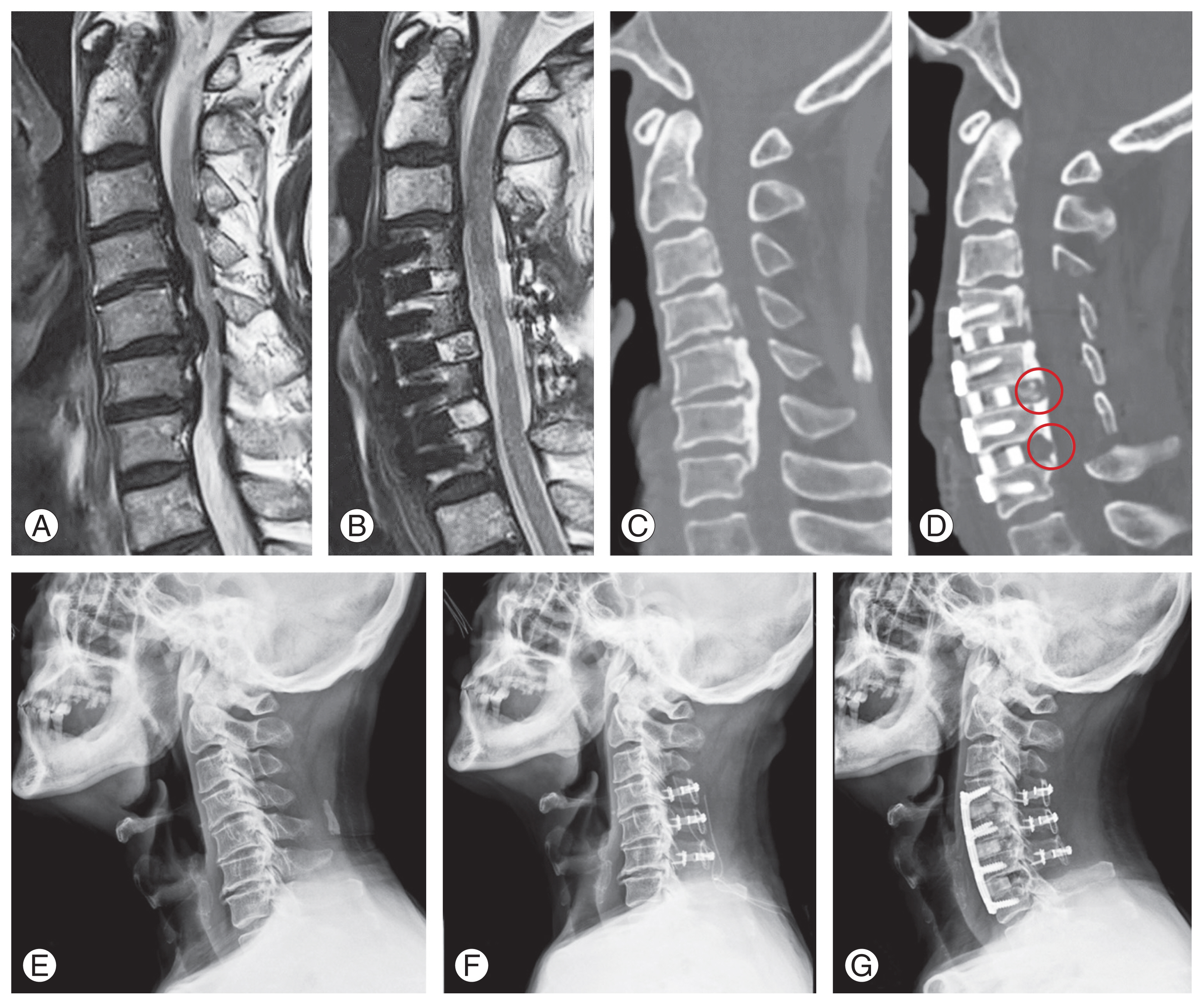
Case analysis following the algorithm of Fig. 2, in a 75-year-old male patient diagnosed with cervical myelopathy due to ossification of the posterior longitudinal ligament (OPLL). (A) Preoperative magnetic resonance imaging (MRI) sagittal image showing a K-line negative status. (B) Postoperative MRI indicating successful decompression and conversion of the K-line to positive. (C) Preoperative computed tomography (CT) sagittal image revealing continuous type OPLL. (D) Postoperative CT sagittal image, showing that the OPLL mass at each level has not been entirely removed but rather thinned, facilitating lordosis restoration via greenstick fractures (indicated by circles). (E) Preoperative cervical plain lateral radiograph displaying kyphotic alignment. (F) Posterior laminoplasty outcome depicted in plain lateral radiograph. (G) Radiograph post secondary anterior approach: anterior cervical discectomy and fusion, demonstrating achieved lordosis restoration.
Conclusions
For DCM cases with ≤3 disease levels, anterior decompression methods such as ACDF, corpectomy, and VBSO can be considered. For patients with a K-line (±) and >3 levels of disease, laminoplasty is generally the preferred initial treatment option, particularly in the absence of neck pain and instability. In instances where fusion is necessary, posterior laminectomy and fusion is recommended.
In cases with dural adhesion, VBSO can be an effective alternative to corpectomy. The greenstick fracture technique is a useful approach for addressing cervical alignment changes in specific cases, such as long, continuous OPLL with a K-line (−) status. When dealing with >3 levels of disease, accompanied by flexible kyphosis, laminectomy with posterior instrumentation is recommended to facilitate K-line alteration. Conversely, in the presence of rigid kyphosis with >3 disease levels, circumferential surgery can be utilized. VBSO can be a valuable technique for reducing the stages of surgery while minimizing complications such as dural tears, CSF leakage, pseudarthrosis, and subsidence.
Overall, an algorithmic approach in surgical scenarios can streamline complex decision-making and guide the selection of appropriate surgical methods. However, our algorithmic approach is merely suggestive and should only serve as a reference tool. Because of the infinite number of potential clinical scenarios, no simple algorithm can account for all the variations. In addition, surgeons have different preferences and skill sets that might favor a different approach than the one described. Therefore, described algorithm should only be used to consider the advantages and disadvantages of each procedure, enabling surgeons to provide tailored treatments for each patient. This approach emphasizes the importance of personalized care in achieving optimal outcomes in the treatment of patients with DCM.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: DHL, KDR; data curation: HRL; formal analysis: DHL; funding acquisition: none; methodology: KDR, HRL; project administration: DHL; visualization: HRL; writing–original draft: HRL; writing–review & editing: KDR, DHL; and final approval of the manuscript: all authors.


