Effect of Thrombin-Containing Local Hemostatics on Postoperative Spinal Epidural Hematoma in Biportal Endoscopic Spinal Surgery
Article information
Abstract
Study Design
Retrospective case–control study.
Purpose
This study aimed to investigate the preventive effect of thrombin-containing local hemostatics (TCLH) on postoperative spinal epidural hematoma (POSEH) in biportal endoscopic spinal surgery (BESS). This study compared the incidence of morphometric and symptomatic POSEH with or without TCLH in BESS.
Overview of Literature
POSEH is reported not uncommon in BESS when compared with conventional spine surgery (CSS). TCLH achieves hemostasis with a high success rate in CSS. However, few studies have examined the effect of TCLH on BESS.
Methods
Patients with and without TCLH were assigned to groups A and B, respectively. POSEH between the two groups was compared morphometrically and symptomatically. The risk factors for symptomatic and morphometric POSEH in BESS were identified.
Results
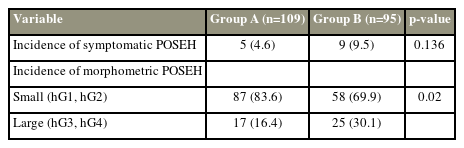
The morphometric POSEH was greater in group B, and the difference was significant (p=0.019). The incidence of symptomatic POSEH was lower in group A with 4.6% (5/109) than in group B with 9.5% (9/95); however, the rate was not significantly different (p=0.136). The morphometric POSEH was classified into two small (hG1 and hG2) and large (hG3 and hG4) and were compared between groups A and B, and the difference was significant (p=0.02). In the multivariable logistic regression, nonuse of TCLH (p=0.004) and preoperative diagnosis of stenosis (p=0.016) were variables found to be significant risk factors of morphometric POSEH.
Conclusions
Severe compression of the thecal sac by POSEH is more common in patients without TCLH. The risk of hematoma formation was higher when bilateral decompression was needed and the cut bone surface was more exposed.
Introduction
Biportal endoscopic spinal surgery (BESS) is a minimally invasive surgical procedure for decompressing various lumbar degenerative diseases, and many studies have reported its versatility and usefulness [1–4]. However, postoperative spinal epidural hematoma (POSEH) is reported not uncommon in BESS when compared with conventional spine surgery (CSS) [5–7]. In BESS, bleeding control is not easy because a saline medium is needed. The use of bone wax for bone bleeding and electric cautery for epidural bleeding is not practical. Thus, a thrombin-containing hemostatic agent would be a good option for bleeding control from inaccessible sites.
Thrombin-containing local hemostatics (TCLH) is an active flowable topical hemostatic agent that contains two independent hemostatic agents, namely, thrombin and calcium chloride solution, and they work together to form a clot at the bleeding site in the surgical field [8]. Many studies have reported that TCLH is effective in achieving hemostasis with a high success rate in CSS [8,9]. However, few studies have investigated the effect of TCLH on BESS. The effect of TCLH may be compromised because BESS is a fluid-based surgery, and TCLH may be diluted and dispersed in the saline medium. Accordingly, we modified the method to apply TCLH under a dry field as much as possible by removing the saline. Accordingly, this study aimed to investigate the preventive effect of TCLH on POSEH in BESS by comparing the incidence of morphometric and symptomatic POSEH with and without TCLH in BESS.
Materials and Methods
1. Study design and participants
This retrospective case–control study was approved by the public institutional review board (POI-2020-0796-001), and the need for written informed consent from the patients was waived. The medical records and postoperative magnetic resonance imaging (MRI) data of patients who underwent lumbar single-level decompression surgery, including laminectomy with or without discectomy, and diagnosed with spinal stenosis or herniated nucleus pulposus (HNP) with the BESS technique under general anesthesia in the authors’ institute between April 2017 and June 2020 were analyzed. All procedures were performed by the senior author (D.K.A.). Patients who underwent revision surgery, had foraminal stenosis or extraforaminal HNP, or had surgery under spinal anesthesia were excluded. POSEH is often identified on postoperative MRI even when adequate hemostasis is achieved, and in some instances, patients may experience severe radiating pain and neurological impairments, although such cases are rare. Proper hemostasis was defined as the absence of active cancellous bone bleeding at the laminectomy site and no active bleeding from epidural vessels, confirmed intraoperatively through the saline infusion, which was halted just before the end of surgery. To reduce the incidence of morphometric and symptomatic POSEH, TCLH has been routinely used for all patients since June 2019. Patients with and without TCLH were assigned to group A (since June 2019) and group B (before June 2019), respectively. The incidence of POSEH was compared between the two groups morphometrically and symptomatically.
2. Data analysis
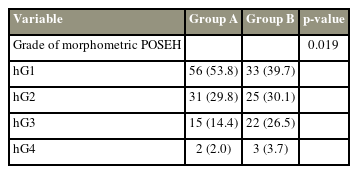
Demographic data, coagulation-related data, diagnosis, and level of operated segments were analyzed to determine homogeneity. Groups A and B included 109 and 95 patients, respectively. Of these, 104 of 109 patients (95%) in group A and 83 of 95 patients (87%) in group B underwent MRI postoperatively. Patients who did not have a postoperative MRI were excluded from the imaging evaluation (Fig. 1). A routine postoperative MRI was taken on day 7. However, if urgent symptoms suggestive of POSEH were present, MRI was taken immediately. On the postoperative MRI, the morphometric measurement of POSEH was obtained from the T2 axial images that had the maximal thecal sac compression site, with a 4-grade morphometric scale as follows: hG1, thecal sac compression less than one quarter; hG2, between one quarter and half; hG3, between half and three-quarters; and hG4, more than three-quarter obstruction (Fig. 2). The neurological state was evaluated using a 5-grade neurological scale as follows: nG0, normal; nG1, unilateral or bilateral leg pain and numbness; nG2, unilateral motor weakness; nG3, bilateral motor weakness; and nG4, cauda equina syndrome. Patients who experienced a neurological deficit that worsened compared with their preoperative state or those who did not have enough improvement in radiating pain as expected and those with a postoperative MRI that was compatible with morphometric POSEH (≥hG3) underwent revision surgery for hematoma evacuation. Among patients who underwent hematoma evacuation, only those who experienced immediate improvement were confirmed as having symptomatic POSEH. All revision surgeries were performed using the BESS technique under local anesthesia. Revision surgery was terminated after confirming immediate improvement of the patient’s symptoms following hematoma evacuation in the operating room. The incidence of symptomatic POSEH and the difference in morphometric POSEH were compared between groups A and B. Finally, the risk factors for symptomatic and morphometric POSEH in BESS were investigated. Statistical analysis was performed using the Student t-test for parametric variables and Fisher’s exact test and chi-square test for nonparametric variables. The likelihood ratio test was applied in cases where the expected frequency was <5. The morphometric comparison of POSEH was performed using the Mann-Whitney U test. For the analysis of risk factors, the multivariable logistic regression test was used. IBM SPSS Statistics ver. 22.0 (IBM Corp., Armonk, NY, USA) was used for all analyses.
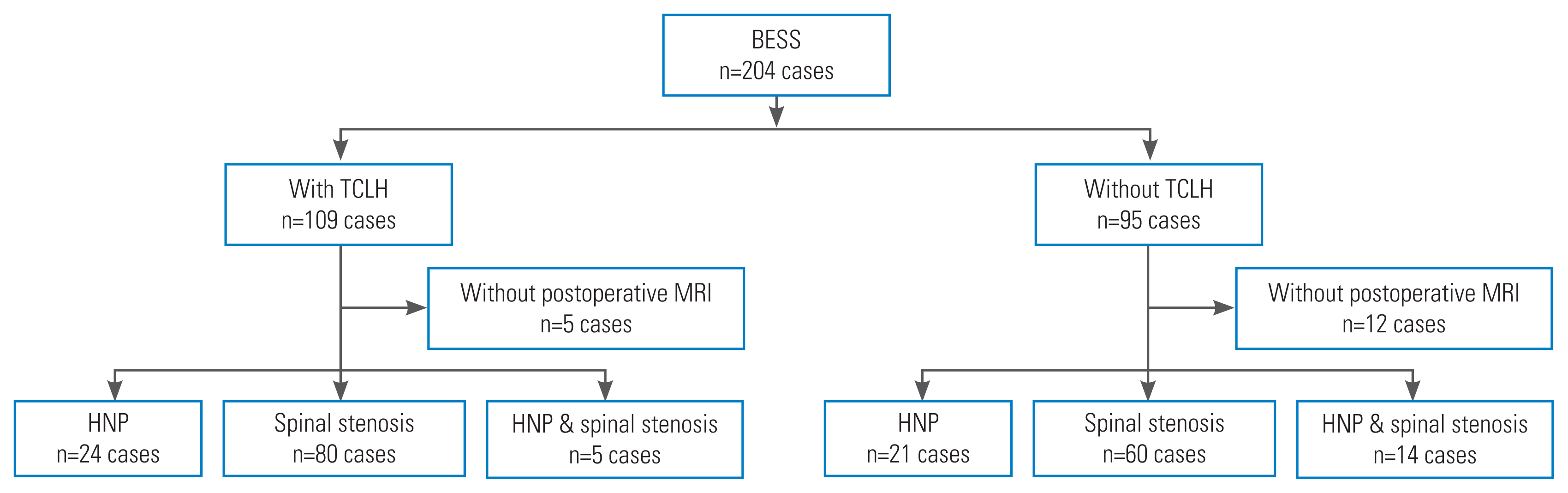
Flow chart of the study subjects. BESS, biportal endoscopic spinal surgery; TCLH, thrombin-containing local hemostatics; MRI, magnetic resonance imaging; HNP, herniated nucleus pulposus.
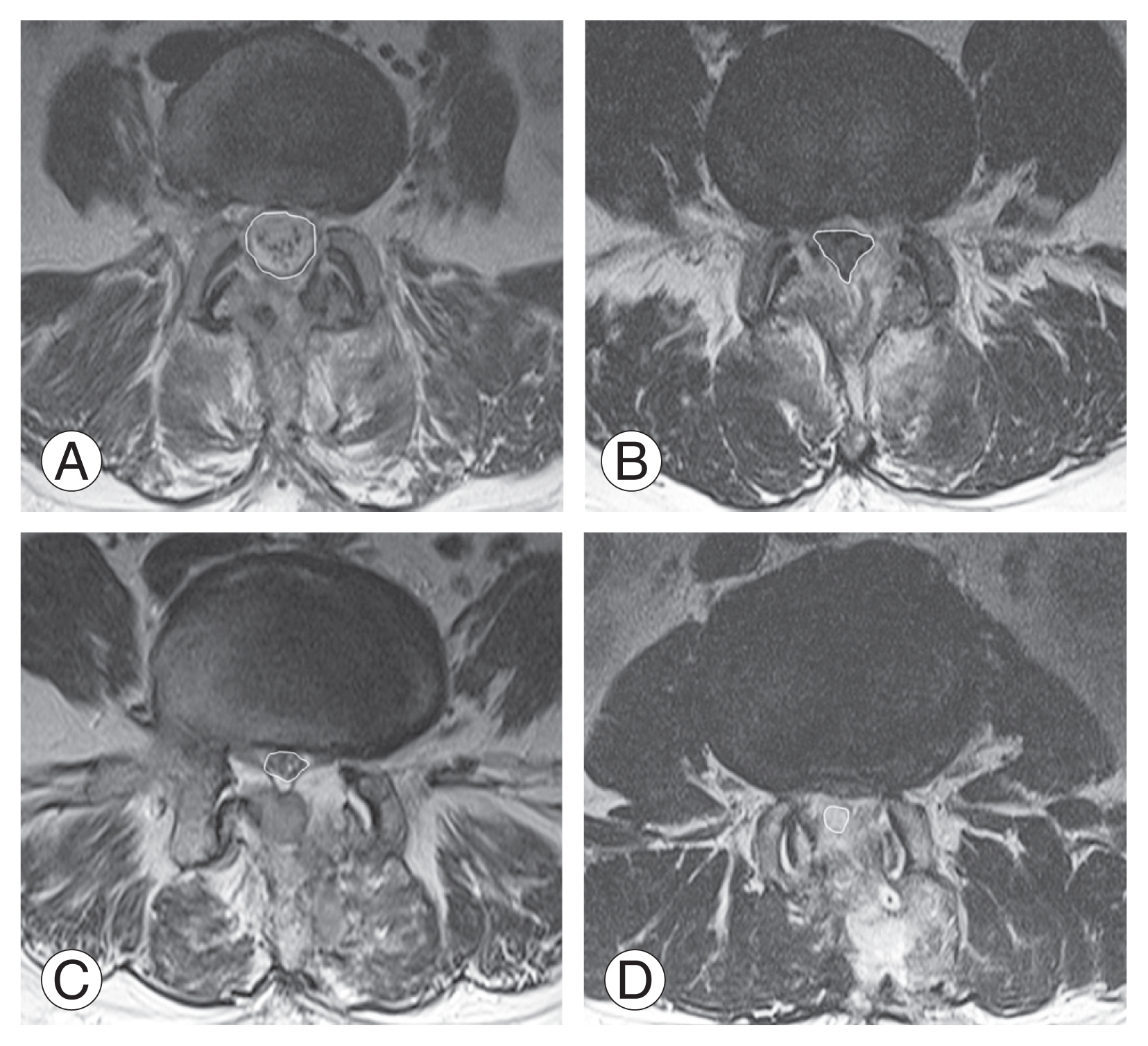
The grading system of thecal sac compression by epidural hematoma in T2 magnetic resonance imaging axial images. (A) Grade 1; that stands for the thecal sac compression less than a quarter. (B) Grade 2; between a quarter and a half. (C) Grade 3; between a half and three quarters. (D) Grade 4; more than three quarters obstruction.
3. TCLH
TCLH (CollaStat, DalimTissen Co. Ltd., Seoul, Korea) was administered over the decompressed epidural space just before the end of surgery. Collastat was used to alleviate the financial burden of patients because the product is covered by the national health insurance in the author’s country. Initially, applying TCLH effectively in the saline-filled surgical field during BESS poses a challenge because the agent would disperse into the saline solution. To overcome this, a dry field was created (Fig. 3). Initially, meticulous bleeding control was performed, and then the saline infusion was discontinued. A nozzle that can reach the epidural space was used to evacuate as much remaining saline as possible. Then, TCLH was instilled under direct endoscopic visualization to the sides of the bleeding area through the nozzle. Gentle manual compression was applied to the skin for approximately 4 minutes, based on the normal bleeding time of approximately 3 minutes [10]. The endoscope was inserted again to confirm hemostasis, and any excessive matrix that was not associated with the clot was removed. Excessive matrix or active thrombin must be removed, as leaving them can lead to POSEH [11].
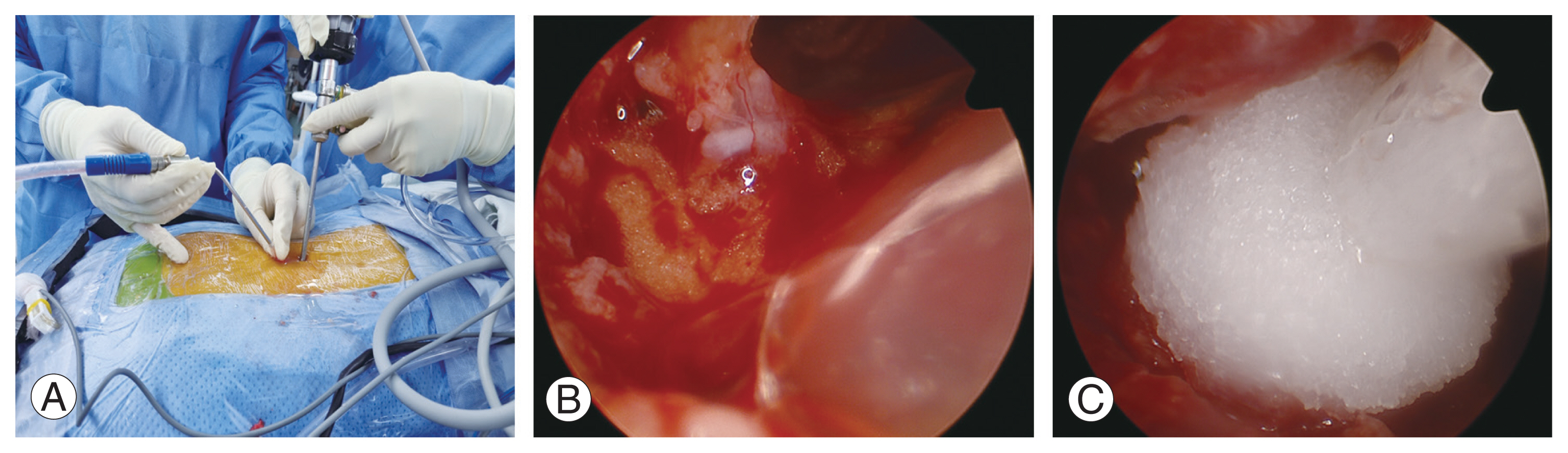
Novel method using thrombin-containing local hemostatics (TCLH) in biportal endoscopic spinal surgery. (A) Nozzle capable of reaching the epidural space was used to evacuate remaining saline. (B) Epidural space after saline evacuation. (C) TCLH was instilled under direct endoscopic visualization to the sides of the bleeding area through the nozzle.
Results
This study enrolled a total of 204 patients, of which 119 (58.3%) were female and 85 (41.7%) were male. Both groups were homogeneous in age, sex, number, and level of the operated segments (Table 1). Morphometric POSEH was hG1 in 56 (53.8%), hG2 in 31 (29.8%), hG3 in 15 (14.4%), and hG4 in 2 (0.2%) cases in group A and hG1 in 33 (39.7%), hG2 in 25 (30.1%), hG3 in 22 (26.5%), and hG4 in 3 (3.6%) cases in group B. It was greater in group B, and the difference was significant (p=0.019) (Table 2). The incidence of symptomatic POSEH was lower in group A with 4.6% (5/109) than in group B with 9.5% (9/95); however, the rate was not significantly different (p=0.136) (Table 3). Of the five patients with symptomatic POSEH in group A, three had hG3 and two had hG4 morphometric POSEH, and of the nine patients with symptomatic POSEH in group B, five had hG3 and four had hG4. For statistical analysis, the morphometric POSEH was classified into small (hG1 and hG2) and large (hG3 and hG4) and were compared between groups A and B (Table 3). Group A had 87 (83.6%) small (hG1 and hG2) and 17 (16.4%) large (hG3 and hG4) cases, and group B had 58 (69.9%) small (hG1 and hG2) and 25 (30.1%) large (hG3 and hG4) cases. The difference was significant (p=0.02). The grade of neurological deficit in group A was nG1 for 4 and nG2 for 1 and that in group B was nG1 for 7, nG2 for 1, and nG3 for 1. The difference was not significant (p=0.518). The three patients who had nG2 and nG3 underwent emergency MRI as soon as their neurological symptoms were confirmed. No risk factors were identified in patients with symptomatic POSEH. For the analysis of the risk factors of the morphometric POSEH, all participants were divided into small (hG1 and hG2) and large (hG3 and hG4) POSEH groups. The nonuse of TCLH (p=0.004) and preoperative diagnosis of stenosis (p=0.016) were variables found to be significant risk factors through multivariable logistic regression (Table 4). All patients who underwent revision surgery for POSEH recovered immediately thereafter, and no neurological sequelae remained.
Discussion
POSEH is one of the complications in BESS [5–7]. The prevention and management of POSEH is of high clinical significance because of the potential for serious neurological deficits. The incidence of MRI-confirmed POSEH following lumbar spine surgery has been reported to be relatively high, ranging from 58% to 89% [12–14]. Fortunately, that of symptomatic POSEH is rare, at 0.1%–1% [12,14–16]. However, most studies only considered cases with serious neurological deficits such as POSEH; therefore, the actual incidence of symptomatic POSEH may be higher than reported. POSEH with at least 50% neural encroachment on postoperative MRI can cause neural injury [5,7], and even a certain degree of epidural hematoma that does not cause neurological symptoms can result in epidural fibrosis and compromise the surgical outcomes [17,18]. Therefore, POSEH must be prevented not only to avoid neurological deficits but also to improve long-term prognosis. Many studies have investigated the incidence and risk factors of POSEH in CSS [13,19]; however, only a few studies have analyzed POSEH after BESS [6]. Some studies have reported a higher incidence of POSEH in BESS than in conventional open surgery [5–7]. Based on the author’s experience, controlling bleeding in a saline-filled surgical field during BESS is more challenging than in CSS because tiny bleeding may be masked by the hydrostatic pressure created by the saline solution. In addition, applying bone wax to the wet bone surface can be difficult, and the use of electrical cautery on the epidural vessels may be challenging because of nerve root stimulation. Despite studies on the POSEH-associated risk factors, the results are not consistent, and most of the factors are not practically avoidable [19–21]. The ideal approach to prevent POSEH is to control bleeding in the final stage of surgery. The authors employed TCLH for this purpose, assuming that it can effectively cover all bleeding points from the muscle, bone surface, and epidural vessels, given that the surgical field in BESS is not wide. TCLH is a hemostatic agent that contains collagen- and bovine-derived thrombin. Collagen binds tightly to bleeding surfaces, providing a matrix for clot formation and strengthening and promoting platelet aggregation, activation, degranulation, and release of clotting factors [22–24]. Thrombin plays a role in clot promotion and fibrin formation. TCLH induces hemostasis at the beginning and end of the coagulation cascade by promoting platelet activation and facilitating fibrin formation [8]. Although TCLH has a volume of 3 mL and a small hemostatic effect through physical compression, compression was routinely performed to increase the contact between the surgical area and TCLH whenever possible.
The study’s primary objective was to determine whether TCLH use during central stenosis or HNP with BESS is associated with a difference in the incidence of symptomatic and morphometric POSEH. In this study, the rates of symptomatic POSEH were 4.6% and 9.5% in groups A and B, respectively, which was higher than those in other studies [5,9]. In other studies, symptomatic POSEH cases were diagnosed only when severe paralysis occurred [5,9]. However, in the present study, if there was a minor neurological deficit or even less improvement of radiating pain than expected and >50% of thecal sac compression was confirmed on postoperative MRI, hematoma removal was performed, which is the reason why MRI was conducted immediately for symptomatic cases and 1 week after surgery for asymptomatic ones. If symptoms improved, all were diagnosed as symptomatic POSEH; thus, the incidence rate was higher than that in other studies. In addition, all these conditions were judged to have clinical significance as immediate symptomatic improvement was confirmed after hematoma removal. We tried to obtain statistical significance by broadening the diagnostic criteria for symptomatic POSEH; however, the difference between the two groups did not reach statistical significance. This is considered a type 2 error caused by the relatively not large sample size. In addition, morphometric POSEH and the degree of compression of the thecal sac by the hematoma were assessed and compared. The 4-scale measurement method used in previous studies was adopted [6]; however, in this study, it was modified and applied in two scales to reduce type 2 errors. As a result, a significant difference between the two groups was confirmed. In addition, secondary observations identified risk factors for the formation of large morphometric POSEH. Multivariable logistic regression analysis identified nonuse of hemostatic agents and spinal stenosis as risk factors. Stenosis has a higher risk than HNP because bilateral laminectomy is required in stenosis, which increases the exposure of the bleeding cut bone surface. In addition, our study included patients who underwent single-level lumbar endoscopic decompression “with or without” discectomy. Endoscopic discectomy often results in a sudden gush of congested blood; thus, the incidence of POSEH may be influenced by whether discectomy was performed. However, since no statistically significant difference was found between the preoperative diagnoses and the demographic data of both groups, the effect of discectomy on POSEH was considered to be similar in both groups. There were some limitations in this study. Above all, statistical significance could not be confirmed in the analysis of symptomatic POSEH because the sample size was not sufficient. Owing to the relatively low incidence of symptomatic POSEH, statistical evidence is expected to be attainable only with a larger sample size, and further studies are currently underway to address this. The study’s inability to establish this correlation is attributed to a type 2 error. Therefore, statistical significance was derived by measuring morphometric POSEH; however, caution is required in clinical application. Considering the learning curve period of the BESS technique, the study was conducted after excluding the initial 50 cases. Although the two groups underwent surgery at different times, all cases were performed by the same surgeon; thus, we thought that the influence of the difference in timing would be minimal. Finally, patients presenting neurological symptoms underwent an early postoperative MRI, resulting in varying postoperative MRI timings. However, as patients who underwent MRI early were confirmed to have neurological symptoms, likely, the size of the hematoma had not decreased even if they were examined after 1 week over time but increased.
Conclusions
In BESS, the incidence of clinically symptomatic POSEH is not uncommon, occurring in 4.6% and 9.5% of patients in whom TCLH was used and not used, respectively, including patients with mild neurological symptoms. After BESS, severe compression of the thecal sac by POSEH occurred more frequently in patients without TCLH. The risk of hematoma formation was higher when bilateral decompression was required, and the cut bone surface was more exposed.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Study design: DKA, YHK. Data collection: YHL, JSJ. Data analysis: JSL. Manuscript writing: YRK, YHK. Proofreading: JSJ. All authors read and approved the final manuscript.