Surgical Outcomes of Full Endoscopic Posterior Cervical Foraminotomy for Proximal Cervical Spondylotic Amyotrophy
Article information
Abstract
Study Design
Retrospective analysis of case series.
Purpose
This study aimed to clarify the effects of full endoscopic posterior cervical foraminotomy (FPCF) on cervical spondylotic amyotrophy (CSA).
Overview of Literature
The method for decompressing the ventral nerve root and anterior horn (AH) in CSA is controversial.
Methods
Patients without myelopathy who underwent FPCF for proximal CSA between 2017 and 2022 were analyzed. The outcome measure was the results of the manual muscle testing (MMT) of the deltoid and biceps. Preoperative nerve root and AH compression were evaluated by magnetic resonance imaging. The intervertebral foramen morphology and bony decompression extent were evaluated by computed tomography.
Results
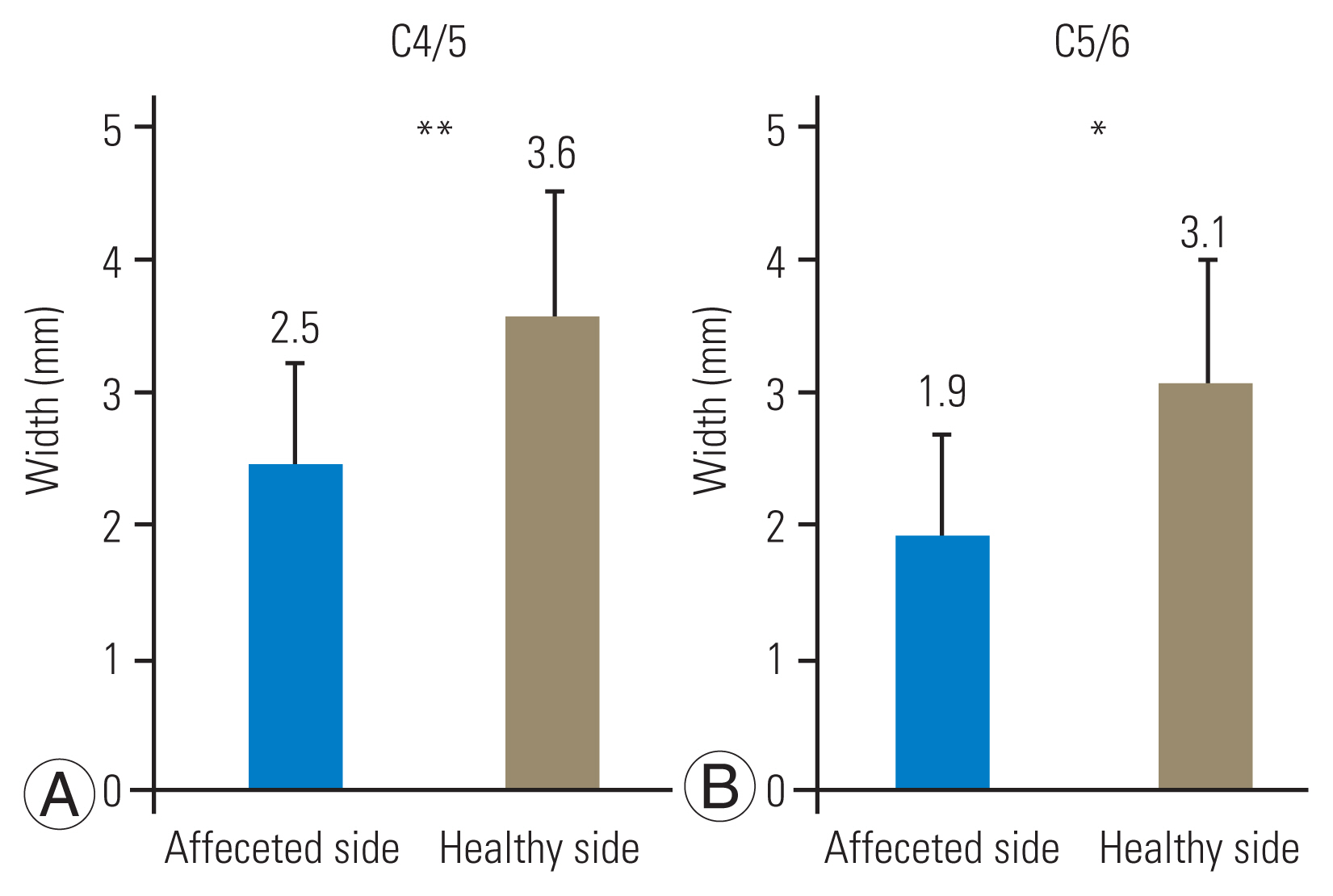
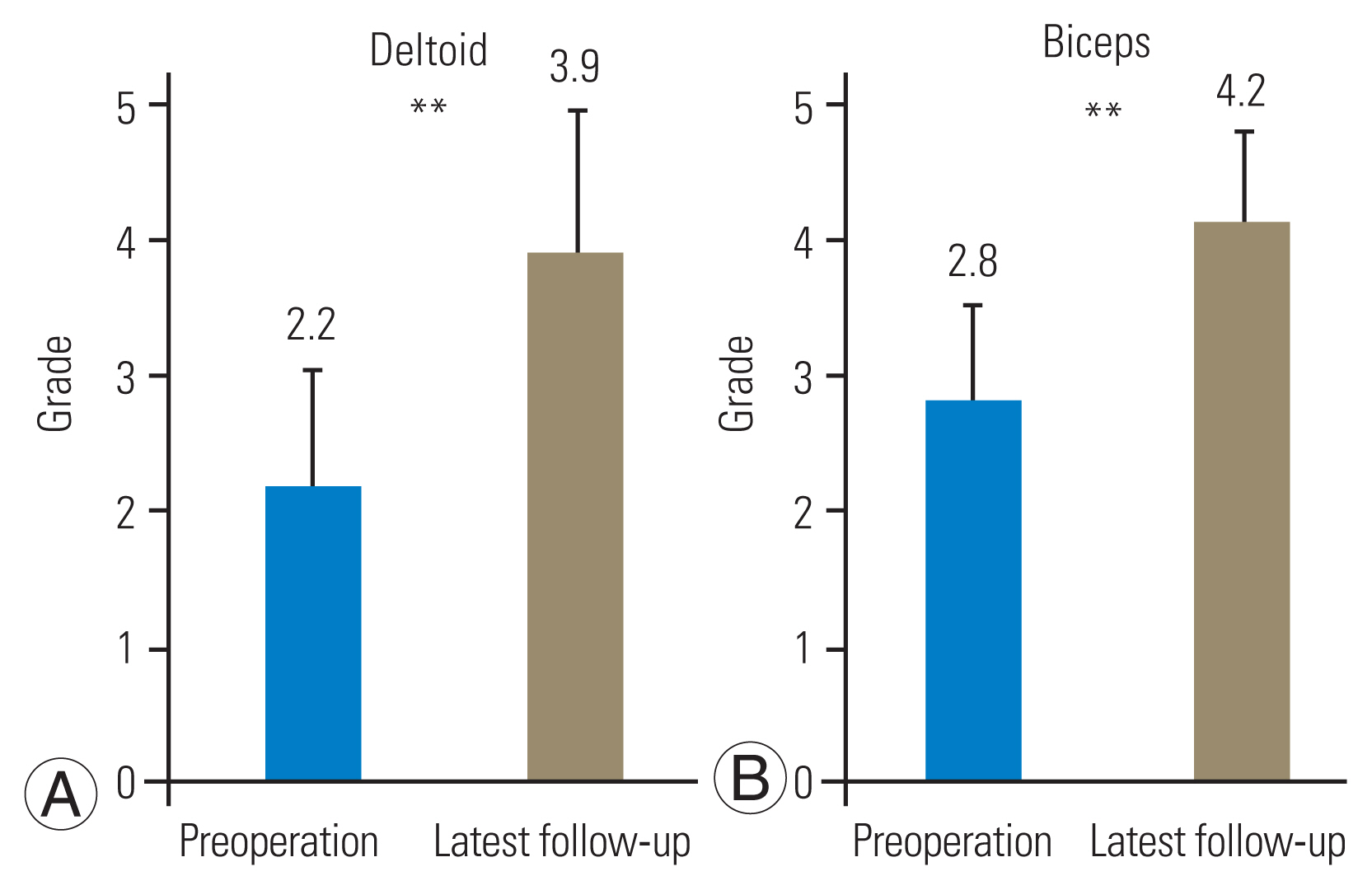
FPCF was performed at the C4/5 level and at the C4/5 and C5/6 levels in 14 and 11 patients, respectively. The width of the narrowest intervertebral foramen was significantly narrower on the affected side than on the healthy side at the C4/5 (2.5 mm vs. 3.6 mm) and operated C5/6 (1.9 mm vs. 3.1 mm) levels. AH compression occurred at the C4/5 and C5/6 levels in 28% and 21% of the patients, respectively. Bony decompression was performed laterally beyond the narrowest foramen at the C4/5 and C5/6 levels in 96% and 91% of the patients, respectively. Compared with patients without AH compression, in those with AH compression, the lamina was resected medially by an average of >1.7 mm and >3.6 mm at the C4/5 and C5/6 levels, respectively. Furthermore, 76% and 81% of the facet joint surfaces were preserved at the C4/5 and C5/6 levels, respectively. Postoperative MMT grade improvement was excellent, good, and fair in 64%, 20%, and 16% of the patients, respectively.
Conclusions
FPCF was effective for treating proximal CSA.
Introduction
Cervical spondylosis, a degenerative change, occasionally causes severe muscle atrophy and weakness in the upper extremities without severe sensory disturbance, and this is known as cervical spondylotic amyotrophy (CSA) [1–3]. The pathophysiology of CSA involves selective ventral nerve root (VNR) damage (encompassing the intradural anterior rootlets or anterior horn [AH] of the spinal cord) secondary to osteophyte-associated compression and ischemia [2,4–7]. Based on the predominant muscles affected, CSA is classified into the proximal (deltoid and biceps) and distal (triceps, forearm, and intrinsic hand muscles) types [8].
Distal CSA is characterized by a higher incidence of multilevel canal stenosis, AH compression, intramedullary intensity changes, and poor outcomes for several treatments [1–3]; however, detailed mechanisms underlying these relationships remain unknown. The predictors of a treatment response for CSA are unclear, and consensus on the optimal treatments is insufficient [1–3,9]. Conservative treatments, such as physical therapy and vitamin B12 supplementation, are reportedly effective for muscular strength recovery in >50% of the patients [2,10]; therefore, preoperative conservative treatment for 1–6 months is a common management strategy for CSA. However, Tauchi et al. [7] recommended early surgical interventions after CSA diagnosis for patients resistant to conservative treatment or those with preoperative severe atrophy. Direct VNR and AH decompression is believed to be achieved by removing the anterior osteophytes through an anterior cervical discectomy and fixation (ACDF) [11,12]. However, in some cases, the direct removal of osteophytes within the intervertebral foramen is difficult in terms of controlling bleeding and securing the visual angle. Thus, a posterior decompressive procedure, i.e., foraminotomy, has been considered an alternative with outcomes comparable to those of ACDF [4,6].
A recent study revealed that minimally invasive posterior foraminotomy (including full endoscopic posterior cervical foraminotomy, FPCF) is as safe and reliable for radiculopathy without cervical myelopathy as conventional ACDF [13]. In this study, the effect of FPCF on CSA treatment was first evaluated. Then, to examine the effect of posterior foraminotomy and full endoscopic spine surgery (FESS) on CSA, foraminotomy was attempted outward to cross the narrowest foramen and focused on proximal CSA.
Materials and Methods
1. Ethics statements
This study was approved by the institutional ethics board of Nippon Koukan Hospital (approval no., 202209) and adhered to the declaration of Helsinki. Information regarding the conduct of research including objectives was disclosed and the subjects were provided an opportunity to refuse inclusion in the research.
2. Study design and population
Patients who underwent FPCF for proximal CSA at the Nippon Koukan Hospital between September 2017 and February 2022 were included. CSA diagnosis was established based on the (1) presence of unilateral muscle atrophy and weakness of the shoulder girdle muscles, (2) absence of significant sensory deficit in C5 and C6 dermatomes, and (3) imaging findings indicative of cervical nerve root compression and bony foraminal stenosis at the C4/5 and C5/6 levels [14]. Patients with cervical disc herniation (CDH) without bony degenerative change, ossification of the posterior longitudinal ligament of the cervical spine, a history of cervical spinal surgery, and symptoms of cervical myelopathy were excluded. Needle electromyography was performed preoperatively to rule out amyotrophic lateral sclerosis or other motor neuron diseases when suspected. Denervation potentials and decreased motor unit potentials were observed in the C5–C6 myotome; however, no abnormalities were observed in the thoracic paraspinal or lower limb muscles [2,4]. A neurologist also ascertained the diagnosis. Rotator cuff tears were ruled out based on physical (biceps palsy and impingement sign of the shoulder joint) and magnetic resonance imaging (MRI) and ultrasound findings.
After CSA diagnosis, upper extremity strength training was provided for at least 1 month; surgery was performed if the manual muscle testing (MMT) result did not improve significantly. Rehabilitation was initiated on postoperative day 2 and continued until the muscle strength recovered fully or plateaued for several months. The patients were also provided with resistance training for muscle strength using electric muscle stimulation, compensatory acquisition training for function recovery in paralyzed muscles, and range of motion training to prevent joint contractures [1,2,15]. All patients were followed up for at least 12 months postoperatively.
3. Data collection
Collected data included age at surgery, preoperative period, and MMT grades for the deltoid and biceps. Improvements in MMT grades for the most atrophic impaired muscles were classified as excellent (more than two grades of recovery or full recovery), good (one grade of recovery), fair (no improvement), and poor (worsening) [4]. Preoperative compression of the nerve roots and AH of the spinal cord was evaluated using axial T2-weighted MRI (static magnetic field strength, 1.5 T) (Fig. 1A). AH compression was considered to involve compression and posterior shift of the paramedian spinal cord with asymmetric deformation [4]. The distance from the midline to the edges of bony decompression was evaluated after FPCF using axial computed tomography (CT) (Fig. 1B–D) [10,16,17]. Preoperative and preoperative images showing the preserved length of the facet joint surface in the axial plane and the lateral mass in the coronal plane were compared.
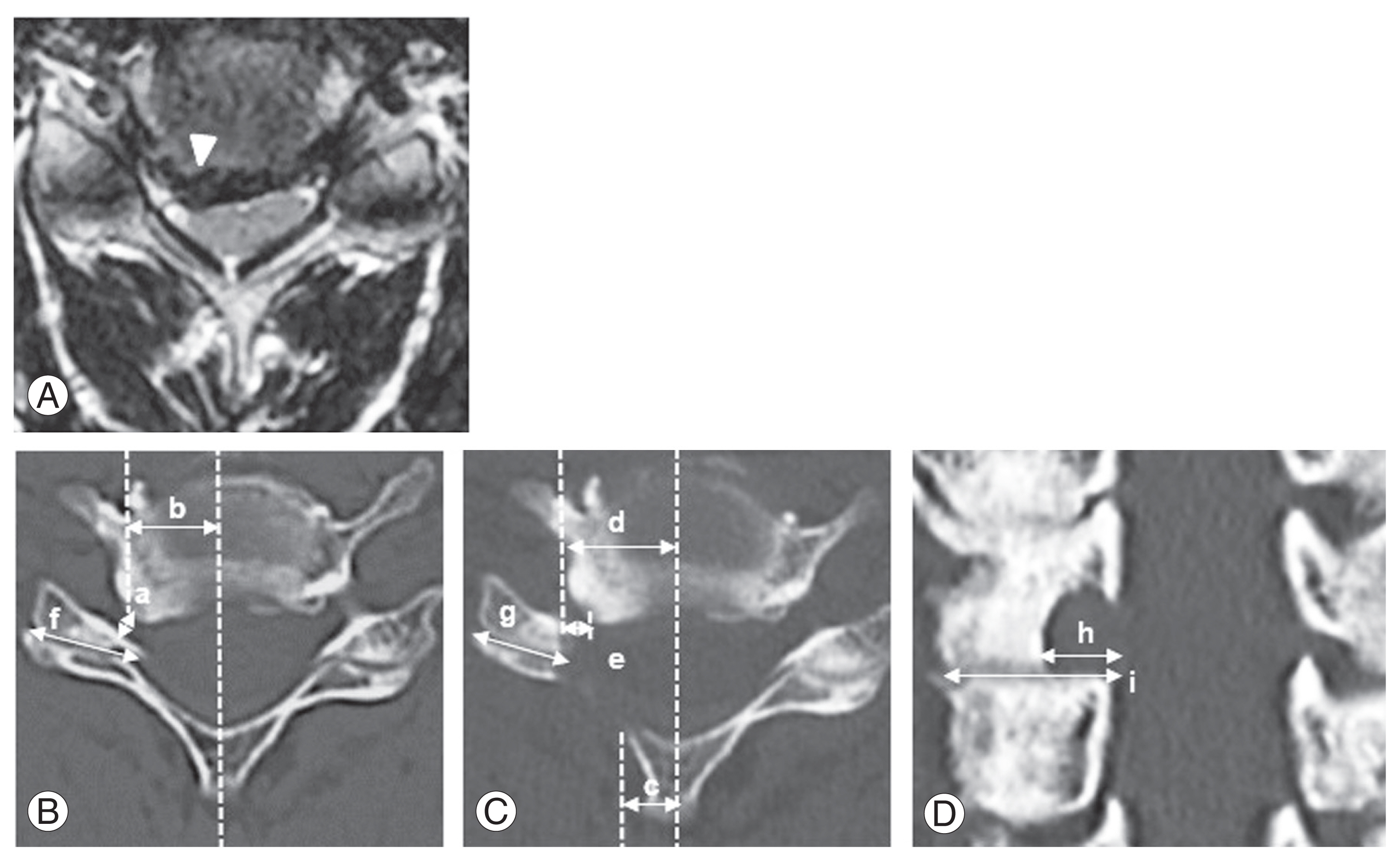
(A) Compression of the nerve root or anterior horn (arrowhead). (B–D) Preoperative width of the narrowest intervertebral foramen (a); distance from the midline to the narrowest part (b); medial (c) and lateral edges (d) of decompression; distance from the narrowest foramen to the lateral edge (e); percentage of preserved facet joint length (g/f×100); and lateral mass length (h/i×100).
4. Full endoscopic posterior cervical foraminotomy
All procedures were performed under general anesthesia while monitoring for motor-evoked potentials of the muscles. An 8-mm skin incision was made at each intervertebral level, and an endoscope (outer diameter, 6.9 mm; angle of vision, 25°; Richard Wolf GmbH, Knittlingen, Germany) was inserted via a beveled-type sleeve at the medial edge of the facet joint (Fig. 2A). A bipolar coagulator system (Elliquence, Baldwin, NY, USA) was used for hemostasis.
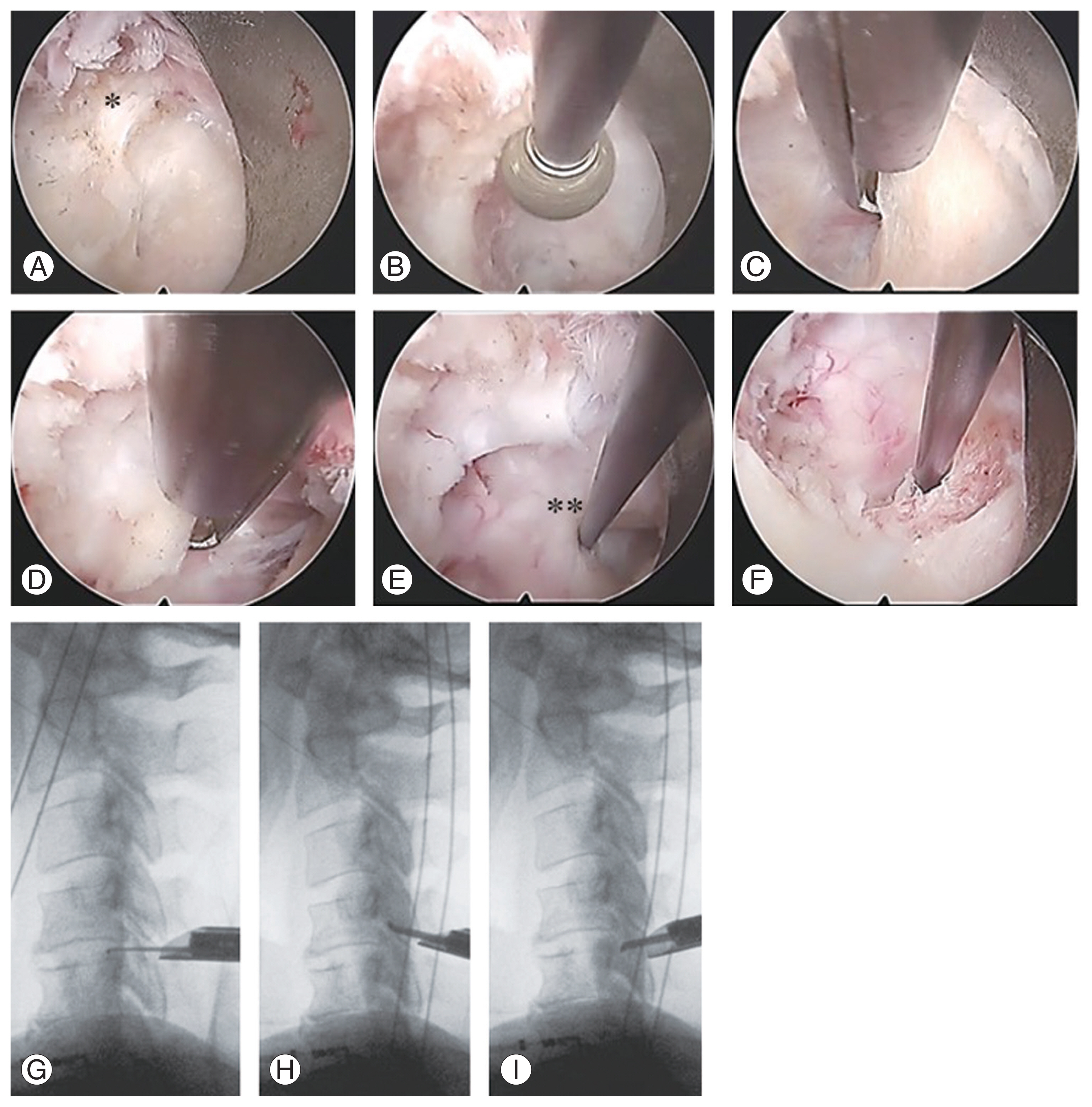
Intraoperative photographs (left: cephalic, right: caudal, upper: medial, and lower: lateral). (A) Interlaminar window and yellow ligament (*). (B) C4 and (C) C5 vertebral laminae were partially drilled. (D) The yellow ligament and perineural membrane are removed. (E) The hook is inserted at the bifurcation of the C5 nerve root (**). (F–I) Nerve root decompression is confirmed within the foramen.
After connective tissue removal, the inferior edge of the upper vertebral lamina and inferior articular process were resected horizontally cephalad and outward from the intersection of the upper and lower laminae using a high-speed drill (NSK, Kanuma, Japan) (Fig. 2B). The superior edges of the lower vertebral lamina and superior articular process were thinly drilled and resected with a Kerrison rongeur until the pedicle was palpable (Fig. 2C). The extent of bony decompression was measured either by the width of the drill tip (3 mm) or by checking the height of the palpable lamina under lateral fluoroscopic view. A sharp spoon was used for slight additional foraminotomy outward. Furthermore, resection of the lamina extended medially in the case of AH compression. After the yellow ligament was detached from the caudal side of the lamina by bony resection, the ligament could be lifted caudally toward the cephalic and medial sides; during this lifting, the perineural membrane was also usually peeled, and the soft tissues were removed carefully (Fig. 2D). Then, the posterolateral aspect of the dura mater and nerve root bifurcation were identified (Fig. 2E). Discectomy can be performed from just below the bifurcation to the lateral side; however, this was not required in any case. Medial traction against the cord was avoided. The endpoint of FPCF was the confirmation of nerve root mobility beyond the narrowest intervertebral foramen under lateral fluoroscopic view (Fig. 2F–I). The wound was closed with drainage, and the patient began walking 3 hours postoperatively without external fixation of the neck [18].
5. Statistical analysis
Differences in average values were assessed using Student t-test and are presented as mean±standard deviations. Differences in percentages were assessed using the chi-square test. Estimate uncertainty was expressed using a 95% confidence interval, and p-values <0.05 were considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Corp., Armonk, NY, USA).
Results
1. Study population
Of the 26 patients in whom FPCF was performed for proximal CSA, 25 (23 men and two women) were included in this study; one male patient was excluded because of a short follow-up period (Table 1). The mean age at surgery was 57 years (range, 21–76 years). The mean preoperative symptom duration was 10 months (range, 1–119 months). The average follow-up period was of 35 months (range, 12–79 months). During the study, laminoplasty with foraminotomy and ACDF were performed in one and two cases, respectively; the patients had cervical myelopathy secondary to severe and/or multiple spinal cord compressions.
2. Preoperative CT and MRI
On CT, the mean width of the narrowest intervertebral foramen was significantly smaller on the affected side than on the healthy side at the C4/5 level (2.5 mm versus 3.6 mm, p<0.001) and operated C5/6 level (1.9 mm versus 3.1 mm, p=0.01) (Fig. 3). These narrowest parts were considered targets of the outward decompression, and the mean distances from the midline to the narrowest foramen were 13.6 mm and 13.7 mm at the C4/5 and C4/5 levels, respectively. Because the foramen was narrow, nerve root compression was observed on the affected side during MRI in all cases. In addition, AH compression was observed in 7 (28%) and 5 (21%) cases at the C4/5 and C5/6 levels, respectively. Patients with cervical myelopathy were excluded from this study, and intramedullary intensity changes, central compression of the cord, and severe spinal canal stenosis were not observed.
3. Operations and postoperative CT
FPCF was performed at the C4/5 level in 14 patients and C4/5 and C5/6 levels in 11 patients. FPCF at the C5/6 level was performed in patients with severely atrophied biceps and foraminal stenosis at this level. The average operative times were 86 minutes (range, 46–141 minutes) and 72 minutes (range, 43–120 minutes) at the C4/5 and C5/6 levels, respectively. In all cases, intraoperative fluoroscopic findings confirmed the palpability of the intervertebral foramen beyond the stenosis. A tiny epineural injury of the nerve root occurred in one case; this was treated conservatively without epidural drain placement. No further complications, such as deterioration of the existing symptoms, infections, spondylodiscitis, and thrombosis, were noted in the remaining cases. The median and average number of postoperative hospitalization days were 2 and 4.2 (range, 2–25), respectively.
Following FPCF, the distances of the lateral edges of the decompression from the midline and the narrowest foramen were 16.4 mm and 2.8 mm at the C4/5 and 16.0 mm and 2.7 mm at the C5/6 levels, respectively. Bony decompression extended outward beyond the narrowest part at the C4/5 and C5/6 levels in 96% and 91% of the cases, respectively (Table 1). Compared with patients without AH compression, the lamina in patients with AH compression was resected medially by an average of >1.7 mm and 3.6 mm at the C4/5 and C5/6 levels, respectively (Fig. 4). The percentages of preserved lateral mass and facet joint surface at the C4/5 level were 53% (range, 43%–63%) and 76% (range, 57%–87%), respectively. The corresponding values at the C5/6 level were 52% (range, 45%–59%) and 81% (range, 64%–92%), respectively (Table 1). More than 60% of the facet surface length was preserved in all cases.
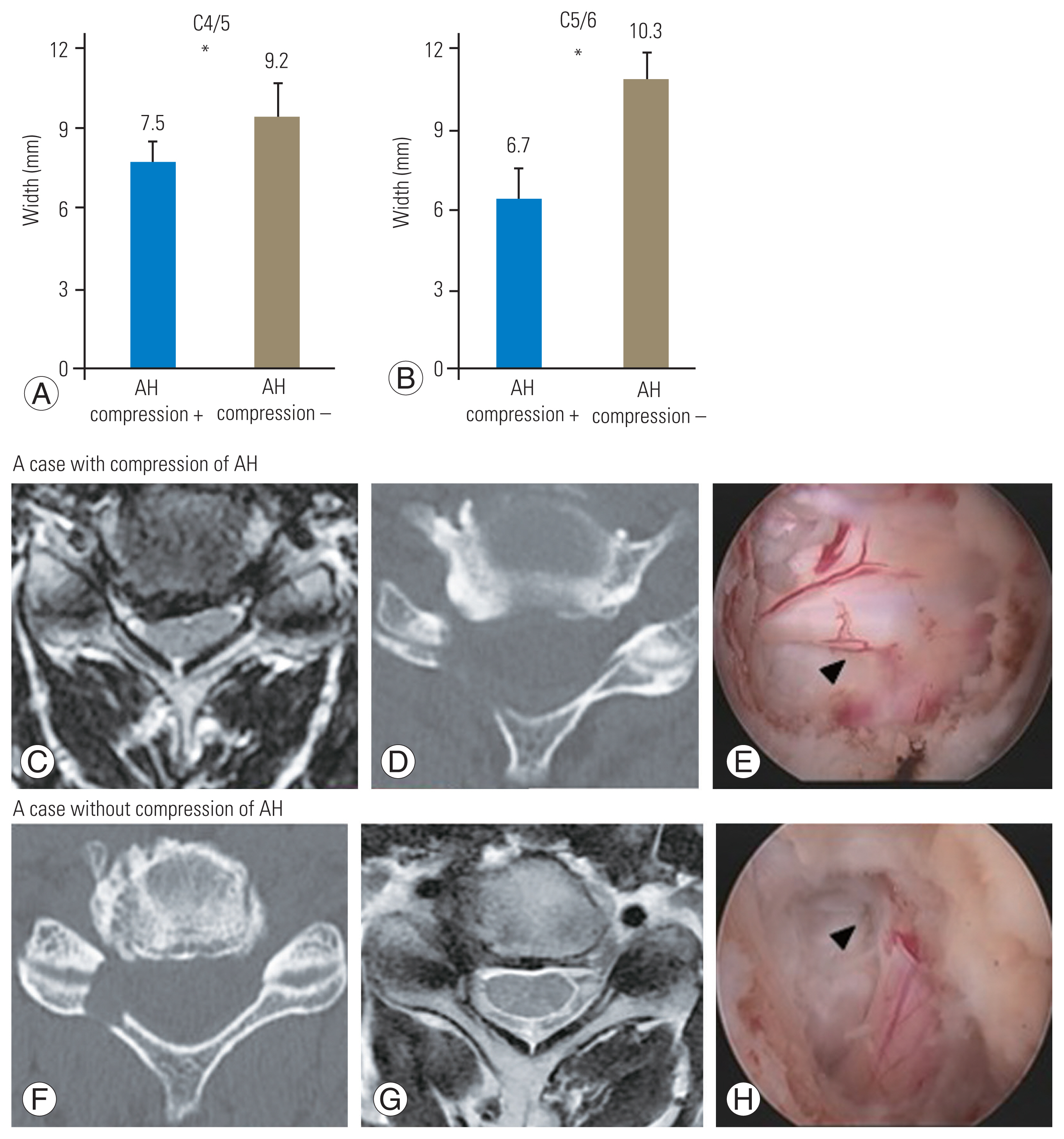
(A, B) Distance of the medial edges of decompression from the midline in cases with and without anterior horn (AH) compression. *p<0.05. (C–H) Representative preoperative magnetic resonance imaging, postoperative computed tomography, and intraoperative surgical field images in the two groups. Arrowheads indicate nerve root bifurcation.
4. Improvement in MMT grades
The MMT grades for the deltoid and biceps improved from 2.2 (range, 0–4) and 2.8 (range, 0–4) preoperatively to 3.9 (range, 2–5) and 4.2 (range, 2–5) at the last observation, respectively (p<0.001 for both) (Fig. 5). Improvement was graded as excellent, good, and fair in 16 (64%), 5 (20%), and 4 (16%) cases, respectively.
Discussion
This study is novel in showing the outcomes of proximal CSA treatment with FESS. FPCF, which confirms nerve root bifurcation from the dura mater and decompresses the nerve root outward to cross the narrowest intervertebral foramen, improved one or more MMT grades in the most atrophic impaired muscles in 84% of the cases.
Regarding the surgical outcomes for proximal CSA, Uchida et al. [12] reported an improvement in the muscle strength with ACDF in 62% of the cases. Fujiwara et al. [4] also reported an improvement in 92% of the patients treated with posterior cervical laminoplasty with or without foraminotomy; they presented a good indication of posterior decompression, particularly in patients with CSA and multilevel spinal stenosis. Although this study did not include cases of cervical myelopathy and our patients’ background differed from those of patients in previous studies, FPCF was as effective for CSA secondary to single or dual nerve root disorders as it is for single-level cervical radiculopathy [13].
In posterior cervical spine surgery, the importance of preserving the deep extensor muscles has been advocated for preventing not only postoperative axial pain but also neck motion restriction because of loss of cervical lordosis [19]. In FPCF, the direct approach of using a dilator through an 8-mm skin incision requires removing the muscle present just on the facet; this is minimally invasive and allows patients to leave their beds and discharge early after surgery. Furthermore, bony stenosis can be eliminated more visibly and safely in the intervertebral foramen while controlling bleeding under a continuous fluid flow, without the risk of developing nonunion and adjacent segment disorders [17,20,21]. Thus, FPCF may best demonstrate the FESS value.
Importantly, 50% of the facet should be preserved to maintain biomechanical stability after a foraminotomy [22]; cervical mobility, alignment, and disc height are reportedly maintained by the facet-preserving FPCF [20,21]. Approximately 75% of the facet joint surface could be preserved by FPCF, which decompresses the foramen laterally beyond the narrowest part. The key FPCF techniques for CSA are similar to those for CSR and CDH [17]. However, the facet width increases from C3 to T1, and its depth decreases from C5 to T1 [23]. Thus, facet preservation and perineural membranous decompression are more difficult at the C4/5 level, where the nerve root bifurcates and enters the intervertebral foramen within a shorter range than at the C5/6 and C6/7 levels. Therefore, performing FPCF for proximal CSA should correspond to the experience of performing FPCF at the C5/6 or C6/7 level or FESS at the lumbar spine, such as the interlaminar method of full endoscopic discectomy wherein laminar and facet joint resections are performed via the posterior approach using a high-speed drill and perineural decompression [24].
Factors associated with poor surgical outcomes in patients with CSA include older age, longer symptom duration, multi-segmental compression, lower preoperative MMT grades, and AH injury [1,7,9,25]. In this study, a statistical analysis on predictors of FPCF outcomes was difficult to perform because of the small sample size; however, some patients showed an excellent recovery, which deviated from the predictions (Table 1). Muscular atrophy secondary to denervation is induced by a complicated reaction of atrophy-inducing factors, such as Murf1 and Atrogin-1 [26,27]. Intramuscular ectopic fat infiltration also occurs during the progression of atrophy, further hastening the reduction in muscle volume and strength [28,29]. Therefore, further studies on optimal surgical procedures and detailed mechanisms underlying muscular atrophy caused by CSA are needed to investigate the treatment strategy.
This study had some limitations. First, this was a retrospective single-center study with a small sample size owing to the rarity of CSA and novelty of FPCF. Second, most patients had already completed their regular visits, and postoperative MRI studies were lacking. A well-designed prospective randomized controlled study with other conventional surgical procedures is warranted to assess the superiority of FPCF.
Conclusions
In conclusion, FPCF could be a candidate for treating proximal CSA, even with AH compression but without severe spinal cord compression and myelopathy.
Notes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
KO designed the study conception, DL drafted the manuscript, DL, TE, and YE collected and analyzed data, and RY revised the manuscript critically. All authors read and approved the final manuscript.
Funding
his study was supported by a Grant for Clinical Research from Nippon Koukan Hospital, and the authors have no competing financial or non-financial interests.