Mini-Open Anterior Lumbar Interbody Fusion Combined with Lateral Lumbar Interbody Fusion in Corrective Surgery for Adult Spinal Deformity
Article information
Abstract
Study Design
Prospective observational study.
Purpose
To introduce the techniques and present the surgical outcomes of mini-open anterior lumbar interbody fusion (ALIF) at the most caudal segments of the spine combined with lateral lumbar interbody fusion (LLIF) for the correction of adult spinal deformity
Overview of Literature
Although LLIF is increasingly used to correct adult spinal deformity, the correction of sagittal plane deformity with LLIF alone is reportedly suboptimal.
Methods
Thirty-two consecutive patients with adult spinal deformity underwent LLIF combined with mini-open ALIF at the L5–S1 or L4–S1 levels followed by 2-stage posterior fixation. ALIF was performed for a mean 1.3 levels and LLIF for a mean 2.7 levels. Then, percutaneous fixation was performed in 11 patients (percutaneous group), open correction with facetectomy with or without laminectomy in 16 (open group), and additional pedicle subtraction osteotomy (PSO) in 5 (PSO group). Spinopelvic parameters were compared preoperatively and postoperatively. Hospitalization data and clinical outcomes were recorded.
Results
No major medical complications developed, and clinical outcomes improved postoperatively in all groups. The mean postoperative segmental lordosis was greater after ALIF (17.5°±5.5°) than after LLIF (8.1°±5.3°, p <0.001). Four patients (12.5%) had lumbar lordosis with a pelvic incidence of ±9° preoperatively, whereas this outcome was achieved postoperatively in 30 patients (93.8%). The total increase in lumbar lordosis was 14.7° in the percutaneous group, 35.3° in the open group, and 57.0° in the PSO group. The ranges of potential lumbar lordosis increase were estimated as 4°–25°, 23°–42°, and 45°–65°, respectively.
Conclusions
Mini-open ALIF combined with LLIF followed by posterior fixation may be a feasible technique for achieving optimal sagittal balance and reducing the necessity of more extensive surgery.
Introduction
The traditional method of correcting adult spinal deformity (ASD) is a posterior-only surgery. However, transforaminal or posterior lumbar interbody fusion (TLIF or PLIF, respectively) is considered insufficient for restoring lumbar lordosis (LL) and sagittal balance [12]. Although osteotomy techniques such as pedicle subtraction osteotomy (PSO) and posterior vertebral column resection can be performed to further correct sagittal balance, these procedures often result in critical complications such as significant epidural bleeding or neurologic deficit [345678].
In response to these limitations, minimally invasive lateral lumbar interbody fusion (LLIF) has been performed in ASD correction, with several studies suggesting reduced complications compared with those of conventional posterior techniques [8910]. Although most studies of LLIF have reported favorable radiographic results after the correction of coronal deformity, the amount of sagittal plane correction was relatively suboptimal, with a 1.6–9.0° increase in LL [91112131415161718]. We postulated that these suboptimal results are attributable to the fact that the L5–S1 levels were not operated or were operated using the posterior fusion technique.
Given that the restoration of LL and sagittal balance is key to the success of adult deformity correction [1920 21], we hypothesized that LLIF alone is insufficient for achieving optimal LL in ASD patients. Thus, we recently performed mini-open anterior lumbar interbody fusion (ALIF) at the most caudal segments (L5–S1 or L4–5, or both) in addition to LLIF to achieve sufficient LL. ALIF has several advantages over TLIF/PLIF or LLIF. It allows direct access to the disk space with a broad surface area for large grafts that can reduce the nonunion rate, and it affords the ability to create a more lordotic angle [222324]. Moreover, the mini-open midline approach to the L5–S1 or L4–S1 levels minimizes blood loss by reducing the need to handle bony or muscular structures. The purpose of this case study was to introduce and present the clinical and radiographic results of mini-open ALIF combined with LLIF for the correction of ASD.
Materials and Methods
1. Study cohort
Thirty-two patients who underwent surgery with ALIF and LLIF for ASD correction between January 2012 and June 2014 were evaluated prospectively after giving informed consent. The minimal follow-up duration was 12.0 months. The inclusion criteria were lumbar degenerative kyphosis (LDK) and lumbar degenerative kyphoscoliosis (LDKS). A coronal Cobb's angle (CCA) of 10° was used as the criterion for distinguishing LDK and LDKS. Patients who had undergone previous lumbar surgery or who had posttraumatic kyphosis were excluded.
2. Surgical procedures
The operations were performed by 2 attending surgeons (C.S.L and S.J.P) in 2 stages with an interval of 1 week. All surgeries included fusion to S1 using bilateral iliac screws. During the first surgery, mini-open ALIF at the L5–S1 or L4–5 levels, or both, was performed, followed by LLIF at these levels. The ALIF procedure was carried out via a retroperitoneal approach after a midline skin incision. After discectomy, the largest possible 8° lordotic-angled polyetheretherketone cage (Syncage; Synthes Inc., West Chester, PA, USA) was fitted. LLIF was performed via a lateral mini-open retroperitoneal approach [25]. Six-degree lordotic-angled polyetheretherketone cages (Clydesdale; Medtronic Inc., Minneapolis, MN, USA) were inserted at each level of the LLIF. The LLIF approach was from the left side in most LDK cases and from the concave side for LDKS cases. The most proximal level of LLIF was L1–2 in 7 patients, L2–3 in 21 patients, and L3–4 in 4 patients. The cages for ALIF and LLIF were filled with allogeneic chip bone grafts mixed with demineralized bone matrix (Grafton; Medtronic Inc.).
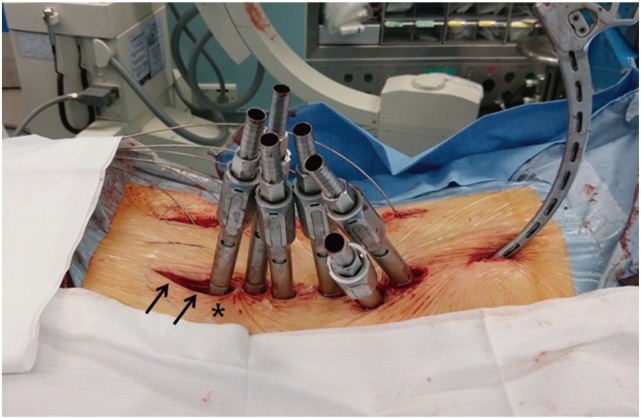
Three days after the first surgery, magnetic resonance imaging and plain radiographs were obtained. The indirect neural decompression shown on the magnetic resonance images obtained after ALIF/LLIF surgery was evaluated to determine the level of decompression for the second surgery. Based on the immediate postoperative LL shown on plain radiographs, the posterior fixation and correction methods were determined according to the amount of LL correction required. The target LL was roughly determined using a simple prediction model according to pelvic incidence (PI) [26]. During posterior instrumentation, percutaneous fixation was performed in 11 patients (percutaneous group) (Fig. 1), open conventional fixation with facetectomy with or without laminectomy was performed in 16 patients (open group) (Fig. 2), and an additional PSO was performed in 5 patients (PSO group). In cases of percutaneous fixation, the most caudal incisions for S1 screw fixation were extended along the posterior superior iliac spine for insertion of the iliac screws and assembly of the rods (Fig. 3).
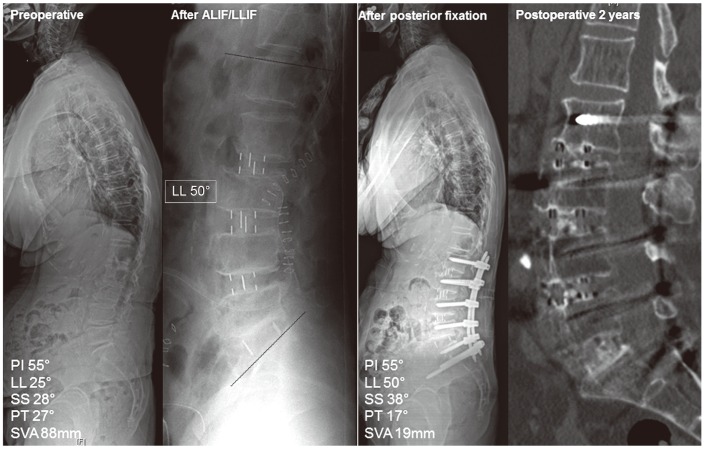
A 75-year-old woman with lumbar degenerative kyphosis underwent anterior lumbar interbody fusion (ALIF) at L5–S1 and lateral lumbar interbody fusion (LLIF) at L2–4. Note that lumbar lordosis (LL) increased by 25° after ALIF and LLIF. During posterior fixation, percutaneous screws were used with iliac screws, and laminectomy was not performed. Computed tomography images obtained 2 years postoperatively show that all segments are well fused. PI, pelvic incidence; SS, sacral slope; PT, pelvic tilt; SVA, sagittal vertical axis.

A 53-year-old woman with lumbar degenerative kyphoscoliosis underwent anterior lumbar interbody fusion at L5–S1, lateral lumbar interbody fusion at L2–4, and open posterior correction. Note that lumbar lordosis (LL) increased from 30° to 59°, and the coronal Cobb's angle (CCA) decreased from 37° to 10° at 1 year postsurgery. PI, pelvic incidence; SS, sacral slope; PT, pelvic tilt; SVA, sagittal vertical axis.
3. Radiographic evaluation
Whole-spine radiographs were evaluated for the following parameters preoperatively, 2 weeks after the final surgery, and at the last follow-up: PI, sacral slope (SS), pelvic tilt (PT), LL, thoracic kyphosis (TK), sagittal vertical axis (SVA), CCA, and segmental lordosis (SL) at each interbody fusion level. LL and CCA were also measured on plain radiographs obtained between the 2 surgeries. Spinopelvic parameters such as PI, SS, PT, LL, TK, and SVA were measured using current standard methods [27]. A PI-LL within 9° was considered successful correction [20]. For SVA, the perpendicular distance from the C7 plumb line to the superoposterior corner of S1 was measured. The sagittal balance was considered negative when the C7 plumb line fell behind the reference point. CCA was defined as the maximal Cobb's angle on the anteroposterior plain radiograph. SL was defined as the angle between the upper endplate of the caudal vertebra and the lower endplate of the cranial vertebra at the level of operation.
4. Hospitalization data and clinical outcomes
Hospitalization data were collected for the following parameters: duration of hospital stay, total surgery time, estimated blood loss, postoperative blood loss, need for red blood cell transfusion, medical or surgery-related complications, and reoperation status. For clinical evaluation, the Oswestry disability index and the visual analog scale score for the back and leg were recorded and compared among the groups.
5. Statistical analysis
Baseline data were compared between the LDK and LDKS groups with the Mann-Whitney U test. Analysis of variance was used to compare parameters among the percutaneous, open, and PSO groups. The Wilcoxon signed rank test was used to compare the overall change in parameters preoperatively and postoperatively. Box and whisker plots provided the first-to-third quartiles of LL increase across the 3 groups. Statistical analysis was performed using SPSS ver. 22.0.0 (IBM Corp., Armonk, NY, USA). A p-values less than 0.05 were considered significant.
Results
The study cohort consisted of 6 men and 26 women aged 67.1±7.4 years (range, 53.0–85.0 years). The preoperative diagnoses were LDK in 17 patients and LDKS in 15 patients. ALIF was performed on 1.3 levels per patient (range, 1–2) and LLIF on 2.6 levels per patient (range, 1–4). During posterior surgery, central decompression was performed for 1.0±1.1 levels per patient and foraminotomy for 0.8±1.2 levels per patient. Total fusion levels were 5.4±2.1 (range, 4.0–14.0). The mean follow-up duration was 15.9 months (range, 12.0–35.6 months).
The number of ALIF levels was greatest in the PSO group followed by the open group and the percutaneous group (1.6±0.5 vs. 1.4±0.5 vs. 1.0, respectively; p=0.015). The number of LLIF levels did not differ among groups (p=0.362). The mean postoperative SL was greater at the ALIF levels than at the LLIF levels (17.5±5.5° vs. 8.1±5.3°, respectively; p<0.001). The mean postoperative increase in SL was also greater at the ALIF levels than at the LLIF levels (11.4±7.9° vs. 4.3±5.3°, respectively; p<0.001).
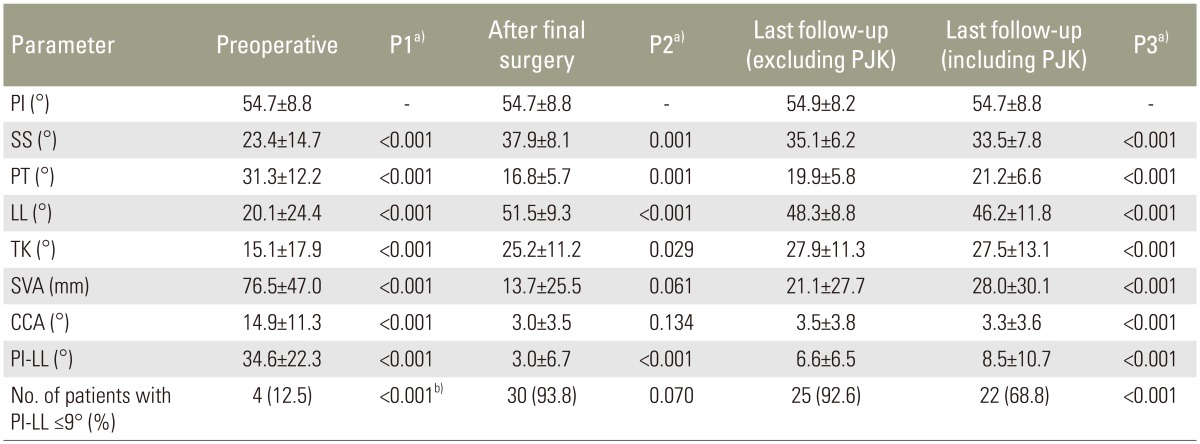
Compared with preoperative values, the sagittal parameters of SS, PT, LL, TK, and SVA were significantly improved after the final surgery (Table 1). The number of patients with PI-LL less than 9° also increased significantly from 4 (12.5%) preoperatively to 30 (93.8%) postoperatively. At the last follow-up, most sagittal parameters were slightly worse than the immediate postoperative parameters, even when 5 patients who developed proximal junctional kyphosis (PJK) were excluded from the evaluation. However, compared with the preoperative data, all parameters in the final follow-up showed improvement, even when the cases of PJK were included. The improvement in the sagittal parameters of SS, PT, LL, TK, and SVA at the last follow-up was significantly greatest in the PSO group followed by the open group and then the percutaneous group. Changes in CCA did not differ significantly among the groups (Table 2).
1. Subanalysis of LL increase
After the first-stage operation with ALIF and LLIF, LL increased from a mean of 20.1° to a mean of 41.9° (p<0.001). The increase in LL after ALIF and LLIF was 13.6°, 25.5°, and 28.1° for the percutaneous, open, and PSO groups, respectively (Fig. 4). After posterior correction, LL did not increase further in the percutaneous group but increased by 9.8° in the open group and 28.8° in the PSO group compared with angles measured after the first-stage operation (Fig. 4). Box and whisker plots demonstrated that the 25%–75% range of LL increase was 4.3°–25.2° for the percutaneous group, 23.2°–41.6° for the open group, and 44.7°–64.9° for the PSO group (Fig. 5).
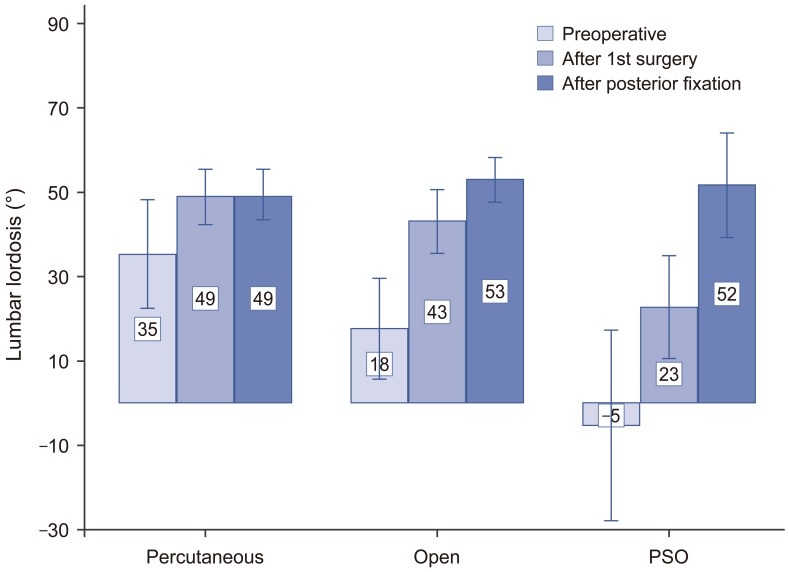
Mean and standard deviation of lumbar lordosis at each designated time in the percutaneous, open, and pedicle subtraction osteotomy (PSO) groups.
2. Hospitalization data and clinical outcomes
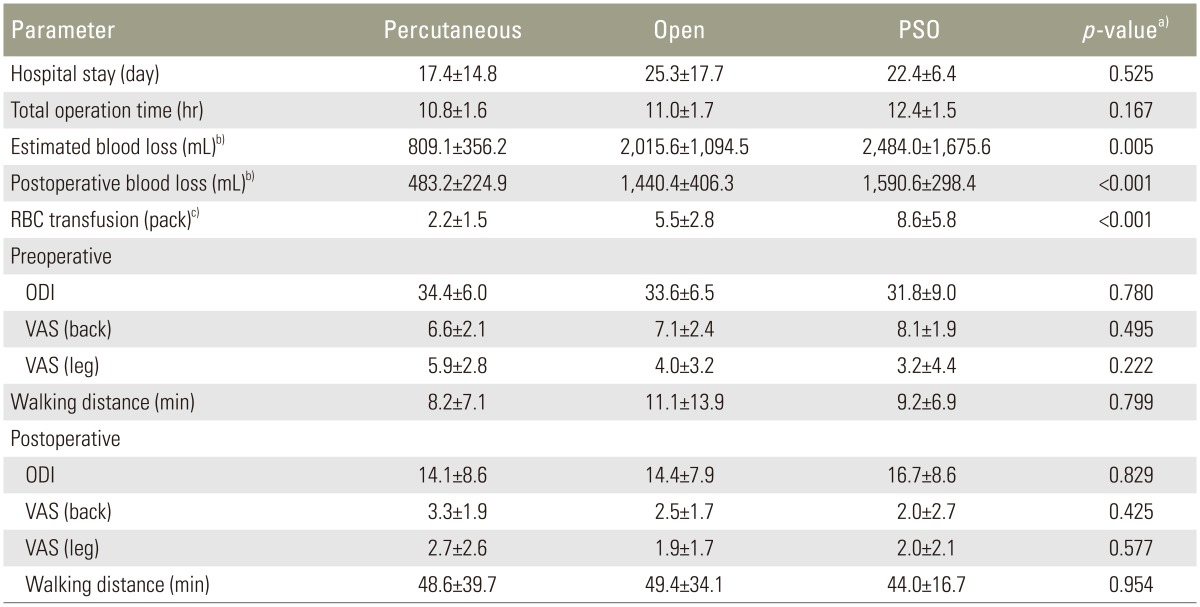
Table 3 lists the detailed hospitalization data for the study participants. Blood loss and subsequent need for transfusion were significantly lowest in the percutaneous group followed by the open group and the PSO group. Compared with preoperative measures, all postoperative clinical outcome measures such as Oswestry disability index, visual analog scale score, and walking distance were significantly improved. Final clinical outcomes did not differ among the posterior fixation groups (Table 3).
In terms of complications, 3 revision surgeries were required to repair epidural hematomas with progressive neurologic deficit in 2 patients (1 in the open group and 1 in the PSO group) and for deep infection in 1 patient (open group). Transient weakness developed in 4 patients (2 in the open group, 2 in the percutaneous group) and persistent weakness in 1 (percutaneous group). During follow-up, PJK developed in 5 patients; 3 of them underwent vertebroplasty, whereas 2 received no treatment. No approach-related complications such as vessel injury or retrograde ejaculation were observed with the exception of 1 case of incisional pseudohernia. No major medical complications occurred.
Discussion
Minimally invasive techniques for corrective surgery for ASD, especially LLIF, have been increasingly used and, compared with the conventional posterior-only approach, have reduced complications [8910]. Although many authors have demonstrated favorable radiographic outcomes after LLIF, the increase in LL is reported to be less than 10° in most studies (Table 4) [9101214151617]. Acosta et al. [15] reported a nonsignificant increase in LL from 42.1° to 46.2°, and the global sagittal alignment also did not change significantly. Similar results have been reported by others. According to a study of pelvic parameters after LLIF by Johnson et al. [16], LLIF does not significantly affect SS, PT, or LL and it increases SL by only 3.3°. Although some authors have demonstrated relatively large increases in LL of 17°–26°, they used hyperlordotic cages (20° and 30°) or performed an anterior release procedure that is not yet commonly available [1128]. We postulated that these suboptimal sagittal plane results might have occurred because the L5–S1 level was not operated or was operated using the posterior fusion techniques.
Because most ASD patients show multilevel degenerations, including those in L5–S1, and subsequent loss of SL at this level, the L5–S1 level is commonly included in ASD surgeries. We performed mini-open ALIF at the most caudal segments (L5–S1 or L4–5, or both) in addition to LLIF to achieve sufficient LL. Compared with TLIF/PLIF or LLIF, ALIF creates greater SL [2224], with TLIF/PLIF of the caudal segments being insufficient to restore sagittal balance in long-fusion surgery [12]. Our results showed that postoperative SL (17.5°) at the ALIF level was approximately twice that at the LLIF level (8.1°). We assumed that the restoration of appropriate SL at the most caudal segments, L5–S1 or L4–S1, is critical for regional sagittal balance because the lordosis at L4–S1 accounts for approximately 70% of the total LL in normal sagittal curvature. ALIF at the caudal segments also has a biomechanical advantage in the correction of global kyphotic curvature because the lever arm of correction becomes longer when the lordosis is produced at a more caudal level. Compared to that at the cranial lumbar segments, the ALIF procedure at the L5–S1 level can be performed more easily because it approaches through the window of the great vessel bifurcation, thereby reducing the need for great vessel manipulation. Additionally, we can expect higher union rate at the L5–S1 level using ALIF compared with TLIF/PLIF, particularly in cases of long-level fusion surgery, because ALIF allows for relatively larger cage sizes.
Despite these well-known advantages of ALIF for ASD correction, the procedure inevitably has potential drawbacks. Because ALIF is performed through a separate incision at a different position, it requires 2 or 3 separate approaches (anterior/posterior or anterior/lateral/posterior), which can prolong the operation time or necessitate 2-stage operations. Two-stage operations might be controversial and burdensome for some surgeons; however, compared with those of posterior-only or 1-stage anterior-posterior surgery, 2-stage anterior-posterior surgery showed better radiological results and lower complication rates in a recent study [29].
ALIF can also be accompanied by approach-related morbidity, including vascular injury, ureteral damage, retrograde ejaculation in men, and abdominal muscle denervation. However, recent studies have reported that the risk of these complications is low [232430]. In the current study, we observed no approach-related morbidities with the exception of pseudohernia related to the LLIF approach, although our sample size was small. Compared with the traditional ALIF technique involving a long incision along the abdomen, mini-open ALIF for L5–S1 can be performed through the linea alba via a midline incision, which might reduce damage to the abdominal musculature and decrease the subsequent risk of abdominal pseudohernia (focal atony of the abdominal musculature).
Our study showed that LL increased from 20° to 42° through the first ALIF and LLIF surgeries. We assumed that the majority of this increase was achieved by ALIF given our results showing greater SL at the ALIF level than at the LLIF level. The mean increases in LL after the first surgery were 13.6°, 25.5°, and 28.1° for the percutaneous, open, and PSO groups, respectively. We attribute these results to the fact that ALIF was performed for the largest number of levels in the PSO group followed by the open group and the percutaneous group (1.6 vs. 1.4 vs. 1.0 levels, respectively), and the results support our assumption that the majority of LL restoration was achieved through ALIF. Percutaneous pedicle screw fixation did not alter LL, whereas open fixation increased LL further. This outcome agrees with the results of a previous study [11].
In assessments of the total increase in LL after posterior fixation, box and whisker plots demonstrated that the range of LL increase was 4.3°–25.2° for the percutaneous group, 23.2°–41.6° for the open group, and 44.7°–64.9° for the PSO group (Fig. 5). From these results, we suggest that if the required amount of LL is less than 25°, the percutaneous method may be adequate to restore sagittal balance. If the required amount is 25°–45°, open posterior correction with facetectomy is sufficient without PSO. However, if the required amount is greater than 45°, corrective techniques such as PSO should be considered. Our study showed that LL increased by a mean of 35° in the open group (Table 2, Fig. 4). PSO would be necessary to obtain this degree of correction with a posterior-only approach. Thus, we believe that our technique can reduce the need for PSO if the required amount of LL is less than 45°.
The main limitations of our study are the small number of patients and the short follow-up duration. We acknowledge that although we chose the posterior fixation methods based on the required amount of LL, we did not use a definite criterion but instead relied on the surgeons' experience because, before this study, there were no data to guide the choice of fixation method based on required LL. Despite these limitations, we successfully demonstrated the utility of ALIF in ASD correction, and the results of this study provide useful suggestions for choosing correction methods that minimize morbidity. Long-term outcomes such as PJK and fusion rate should be investigated further.
Conclusions
Mini-open ALIF combined with LLIF followed by posterior fixation may be a feasible technique for achieving optimal sagittal balance and reducing the necessity of more extensive surgery.
Notes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.